Author:
Mark Sanchez
Date Of Creation:
3 January 2021
Update Date:
1 July 2024

Content
- Steps
- Method 1 of 2: Determining Mass Percentage Based on Specified Weights
- Method 2 of 2: Determining Mass Percentage When No Masses Are Specified
Mass percent specifies the percentage of elements in a chemical compound. To find the mass percentage, it is necessary to know the molar mass (in grams per mole) of the elements included in the compound or the number of grams of each component required in order to obtain a given solution.The mass percentage is calculated quite simply: it is enough to divide the mass of the element (or component) by the mass of the entire compound (or solution).
Steps
Method 1 of 2: Determining Mass Percentage Based on Specified Weights
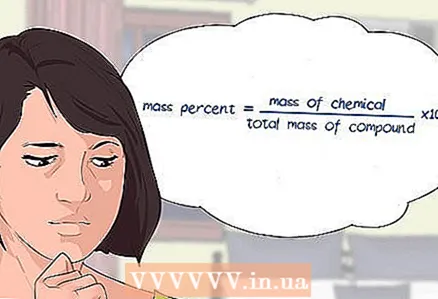 1 Select an equation to determine the weight percent of a chemical compound. The mass percentage is found using the following formula: mass percentage = (mass of component / total mass of compound) x 100. To obtain percent, the result of division is multiplied by 100.
1 Select an equation to determine the weight percent of a chemical compound. The mass percentage is found using the following formula: mass percentage = (mass of component / total mass of compound) x 100. To obtain percent, the result of division is multiplied by 100. - At the beginning of solving the problem, write down the equality: mass percentage = (mass of component / total mass of compound) x 100.
- The mass of the component you are interested in should be in the problem statement. If no mass is given, skip to the next section, which explains how to determine mass percentage with unknown mass.
- The total mass of a chemical compound is found by adding the masses of all elements (components) that are part of this compound (or solution).
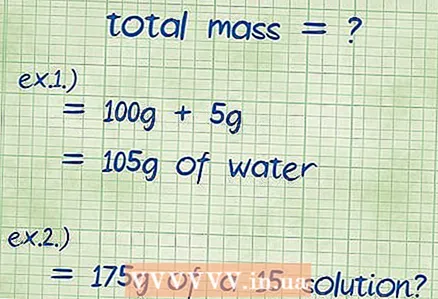 2 Calculate the total mass of the compound. If you know the masses of all the components that make up the compound, just add them up, and this way you will find the total mass of the resulting compound or solution. You use this mass as the denominator in your equation for mass percent.
2 Calculate the total mass of the compound. If you know the masses of all the components that make up the compound, just add them up, and this way you will find the total mass of the resulting compound or solution. You use this mass as the denominator in your equation for mass percent. - Example 1: What is the mass percentage of 5 grams of sodium hydroxide dissolved in 100 grams of water?
- The total mass of the solution is equal to the sum of the amount of sodium hydroxide and water: 100 g + 5 g gives 105 g.
- Example 2: How much sodium chloride and water do you need to make 175 grams of a 15 percent solution?
- In this example, the total mass and the required percentage are given, and it is required to find the amount of substance that needs to be added to the solution. The total weight is 175 grams.
- Example 1: What is the mass percentage of 5 grams of sodium hydroxide dissolved in 100 grams of water?
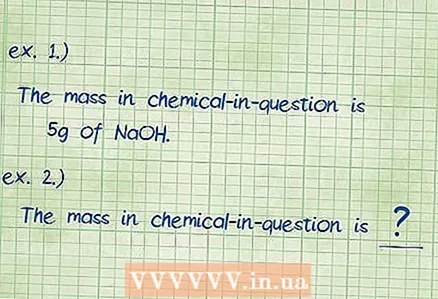 3 Determine the mass of the specified component. If you are asked to calculate "mass percent", you should find out how many percent of the total mass of a substance is the mass of a certain component. Record the mass of the specified component. This will be the numerator in the formula for the mass percentage.
3 Determine the mass of the specified component. If you are asked to calculate "mass percent", you should find out how many percent of the total mass of a substance is the mass of a certain component. Record the mass of the specified component. This will be the numerator in the formula for the mass percentage. - Example 1: The mass of a given component - sodium hydrochloride - is 5 grams.
- Example 2: In this example, the mass of the given component is unknown and must be found.
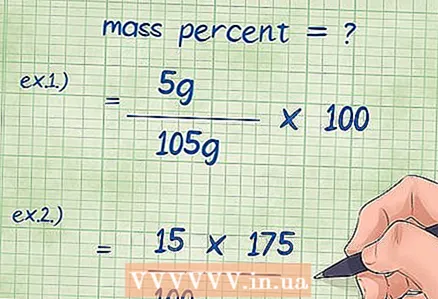 4 Plug in the values into the mass percent equation. After you determine all the required values, plug them into the formula.
4 Plug in the values into the mass percent equation. After you determine all the required values, plug them into the formula. - Example 1: mass percent = (component mass / total compound mass) x 100 = (5 g / 105 g) x 100.
- Example 2: it is necessary to transform the formula for the mass percent so that the unknown mass of the chemical component can be found: mass of the component = (mass percent * total mass of the compound) / 100 = (15 * 175) / 100.
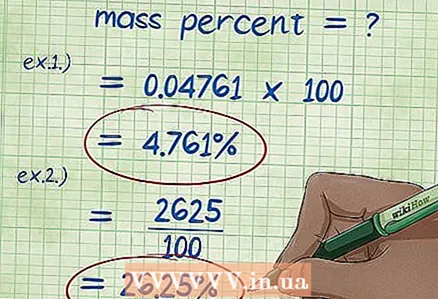 5 Calculate the mass percentage. After substituting all values in the formula for mass percent, perform the necessary calculations. Divide the weight of a component by the total weight of the chemical compound or solution and multiply by 100. The result is the weight percent of that component.
5 Calculate the mass percentage. After substituting all values in the formula for mass percent, perform the necessary calculations. Divide the weight of a component by the total weight of the chemical compound or solution and multiply by 100. The result is the weight percent of that component. - Example 1: (5/105) x 100 = 0.04761 x 100 = 4.761%. Thus, the weight percent of 5 grams of sodium hydrochloride dissolved in 100 grams of water is 4.761%.
- Example 2: The rewritten expression for the mass percentage of a component is (mass percentage * total mass of the substance) / 100, from which we find: (15 * 175) / 100 = (2625) / 100 = 26.25 grams of sodium chloride.
- We find the required amount of water by subtracting the mass of the component from the total mass of the solution: 175 - 26.25 = 148.75 grams of water.
Method 2 of 2: Determining Mass Percentage When No Masses Are Specified
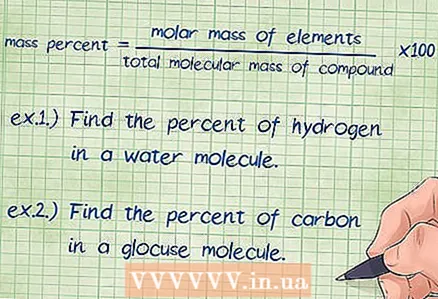 1 Select a formula for the weight percent of a chemical compound. The basic equation for finding mass percent is as follows: mass percent = (molar mass of an element / total molecular mass of a compound) x 100. Molar mass of a substance is the mass of one mole of a given substance, while molecular mass is the mass of one mole of the entire chemical. connections. The division is multiplied by 100 to get the percentages.
1 Select a formula for the weight percent of a chemical compound. The basic equation for finding mass percent is as follows: mass percent = (molar mass of an element / total molecular mass of a compound) x 100. Molar mass of a substance is the mass of one mole of a given substance, while molecular mass is the mass of one mole of the entire chemical. connections. The division is multiplied by 100 to get the percentages. - At the beginning of solving the problem, write down the equality: mass percent = (molar mass of element / total molecular mass of compound) x 100.
- Both quantities are measured in grams per mole (g / mol).
- If you are not given masses, the mass percentage of an element in a given substance can be found using molar mass.
- Example 1: Find the mass percentage of hydrogen in a water molecule.
- Example 2: Find the mass percentage of carbon in a glucose molecule.
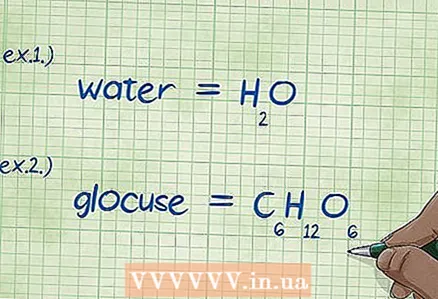 2 Write down the chemical formula. If the example does not give the chemical formulas of the specified substances, you should write them down yourself. If the task contains the necessary formulas for chemical substances, you can skip this step and go directly to the next step (find the mass of each element).
2 Write down the chemical formula. If the example does not give the chemical formulas of the specified substances, you should write them down yourself. If the task contains the necessary formulas for chemical substances, you can skip this step and go directly to the next step (find the mass of each element). - Example 1: Write down the chemical formula of water, H2O.
- Example 2: Write down the chemical formula of glucose, C6H12O6.
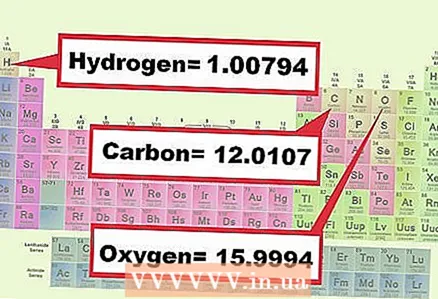 3 Find the mass of each element in the compound. Determine the molar weight of each element in the chemical formula according to the periodic table. Typically, the mass of an element is indicated under its chemical symbol. Write down the molar masses of all the elements that make up the compound in question.
3 Find the mass of each element in the compound. Determine the molar weight of each element in the chemical formula according to the periodic table. Typically, the mass of an element is indicated under its chemical symbol. Write down the molar masses of all the elements that make up the compound in question. - Example 1: Find the molar masses of oxygen (15.9994) and hydrogen (1.0079).
- Example 2: Find the molar masses of carbon (12.0107), oxygen (15.9994) and hydrogen (1.0079).
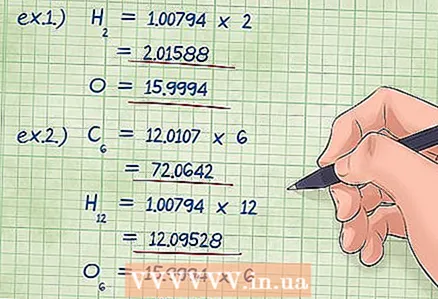 4 Multiply the molar mass of each element by its molar fraction. Determine how many moles of each element are contained in a given chemical, that is, the mole fractions of the elements. Mole fractions are given by the numbers at the bottom of the element symbols in the formula. Multiply the molar mass of each element by its molar fraction.
4 Multiply the molar mass of each element by its molar fraction. Determine how many moles of each element are contained in a given chemical, that is, the mole fractions of the elements. Mole fractions are given by the numbers at the bottom of the element symbols in the formula. Multiply the molar mass of each element by its molar fraction. - Example 1: there is 2 under the hydrogen symbol, and 1 under the oxygen symbol (equivalent to the absence of a number). Thus, the molar mass of hydrogen should be multiplied by 2: 1.00794 X 2 = 2.01588; we leave the molar mass of oxygen the same, 15.9994 (that is, multiply by 1).
- Example 2: under the symbol of carbon is 6, under hydrogen 12 and under oxygen 6. Multiplying the molar masses of the elements by these numbers, we find:
- carbon: (12.0107 * 6) = 72.0642
- hydrogen: (1.00794 * 12) = 12.09528
- oxygen: (15.9994 * 6) = 95.9964
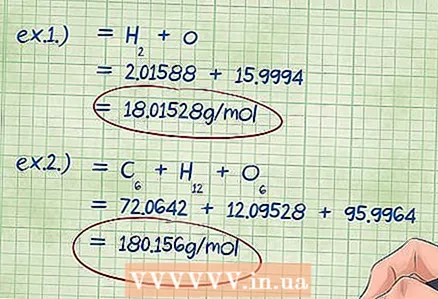 5 Calculate the total mass of the compound. Add up the found masses of all the elements included in this compound. The sum of the molar masses of the elements multiplied by the mole fractions will give you the total mass of the chemical compound. This number is the divisor in the formula for mass percent.
5 Calculate the total mass of the compound. Add up the found masses of all the elements included in this compound. The sum of the molar masses of the elements multiplied by the mole fractions will give you the total mass of the chemical compound. This number is the divisor in the formula for mass percent. - Example 1: Add to 2.01588 g / mol (the mass of two moles of hydrogen atoms) 15.9994 g / mol (the mass of one mole of oxygen atoms), the result is 18.01528 g / mol.
- Example 2: Add the found molar masses: carbon + hydrogen + oxygen = 72.0642 + 12.09528 + 95.9964 = 180.156 g / mol.
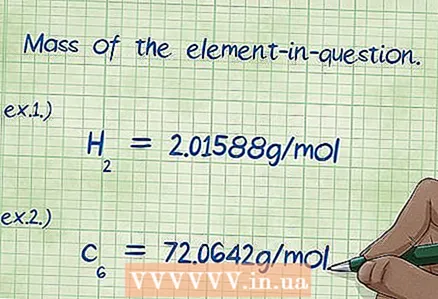 6 Determine the mass of the element of interest. If you are asked to find the "mass percentage", then you should calculate the mass of a certain element that is part of the compound, as a percentage of the total mass of all elements. Find the mass of a given element and write it down. To do this, it is necessary to multiply the molar mass of the element by its molar fraction. As a result, you get the value in the numerator of the formula for the mass percentage.
6 Determine the mass of the element of interest. If you are asked to find the "mass percentage", then you should calculate the mass of a certain element that is part of the compound, as a percentage of the total mass of all elements. Find the mass of a given element and write it down. To do this, it is necessary to multiply the molar mass of the element by its molar fraction. As a result, you get the value in the numerator of the formula for the mass percentage. - Example 1: The mass of hydrogen in the compound is 2.01588 g / mol (the mass of two moles of hydrogen atoms).
- Example 2: The mass of carbon in the compound is 72.0642 g / mol (the mass of six moles of carbon atoms).
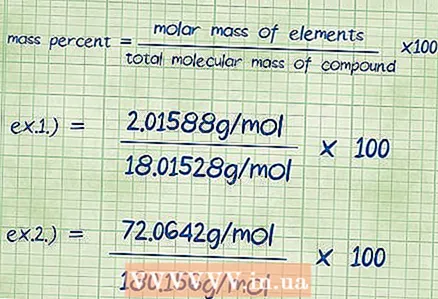 7 Substitute numerical values into the mass percent equation. After you determine the values of all quantities, plug them into the formula that was given in the first step: mass percentage = (molar mass of the element / total molecular mass of the compound) x 100.
7 Substitute numerical values into the mass percent equation. After you determine the values of all quantities, plug them into the formula that was given in the first step: mass percentage = (molar mass of the element / total molecular mass of the compound) x 100. - Example 1: mass percent = (molar mass of element / total molecular mass of compound) x 100 = (2.01588 / 18.01528) x 100.
- Example 2: mass percent = (molar mass of element / total molecular mass of compound) x 100 = (72.0642 / 180.156) x 100.
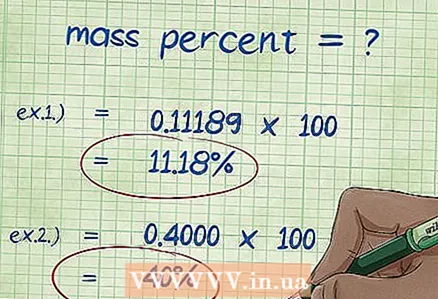 8 Calculate the mass percentage. After substituting numerical values, perform the required arithmetic operations.Divide the mass of the element by the total mass of the compound and multiply by 100. The result is the mass percentage of the element.
8 Calculate the mass percentage. After substituting numerical values, perform the required arithmetic operations.Divide the mass of the element by the total mass of the compound and multiply by 100. The result is the mass percentage of the element. - Example 1: mass percent = (molar mass of element / total molecular mass of compound) x 100 = (2.01588 / 18.01528) x 100 = 0.111189 x 100 = 11.18%. Thus, the mass percentage of hydrogen atoms in a water molecule is 11.18%.
- Example 2: mass percent = (molar mass of element / total molecular mass of compound) x 100 = (72.0642 / 180.156) x 100 = 0.4000 x 100 = 40.00%. Thus, the weight percent of carbon atoms in the glucose molecule is 40.00%.