Author:
Janice Evans
Date Of Creation:
1 July 2021
Update Date:
1 July 2024

Content
- Steps
- Part 1 of 3: Determining the pH Level
- Part 2 of 3: Using Techniques to Lower the pH
- Part 3 of 3: When to Lower Soil pH
- Tips
- Warnings
In chemistry, pH is an index that indicates how acidic or alkaline a given substrate is. The pH values range from 0 to 14: if the pH value is approximately 0, this indicates a very acidic environment, if it approaches 14, it is alkaline. A pH value of 7 indicates a neutral environment. In horticulture and horticulture, the pH of the soil in which plants are grown can have a serious impact on plant growth and health. While most plants grow well at a pH of 6.5-7, there are species that do much better at a certain soil acidity, so serious gardeners should learn the basics of soil acidity management. Start with the first step and you will learn how to lower the pH of the soil in your garden.
Steps
Part 1 of 3: Determining the pH Level
 1 Check the soil pH level. Before you add anything to the soil to change the acidity level, be sure to check how much its pH is different from what you need. You can purchase a DIY pH kit from your gardening store, or see if you can order a soil test from a professional.
1 Check the soil pH level. Before you add anything to the soil to change the acidity level, be sure to check how much its pH is different from what you need. You can purchase a DIY pH kit from your gardening store, or see if you can order a soil test from a professional.  2 Dig 5 small holes in the area. The easiest way to determine your soil pH is with a pH test kit. These kits are usually inexpensive and are available at many hardware and gardening stores.Start by taking soil samples from the area where you want to test the pH. Dig five small pits, 15-20 cm deep. The location of the pits should be random within the site - this will give you the "average" pH of your soil. You won't need the soil you got out of the holes now.
2 Dig 5 small holes in the area. The easiest way to determine your soil pH is with a pH test kit. These kits are usually inexpensive and are available at many hardware and gardening stores.Start by taking soil samples from the area where you want to test the pH. Dig five small pits, 15-20 cm deep. The location of the pits should be random within the site - this will give you the "average" pH of your soil. You won't need the soil you got out of the holes now. - Please note that we are only providing the most general instructions in this section - you will need to follow the instructions that came with your pH kit.
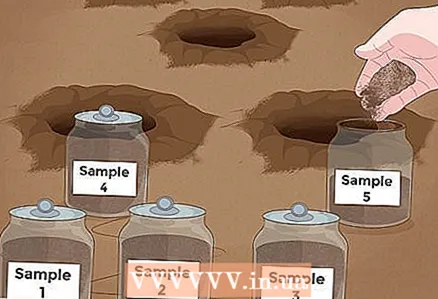 3 Take a soil sample from each hole. So, take a bayonet or shovel and cut a narrow "slice" of soil from the side of each hole. This "slice" should be a half moon, 1.3 cm thick. Place the samples in a clean, dry basket.
3 Take a soil sample from each hole. So, take a bayonet or shovel and cut a narrow "slice" of soil from the side of each hole. This "slice" should be a half moon, 1.3 cm thick. Place the samples in a clean, dry basket. - Try to take enough soil from each hole so that the total sample volume is approximately 0.94 liters or more. For most methods, this is sufficient.
 4 Mix the soil in a basket and scatter a thin layer over newspaper to dry. Leave the soil to dry out until it feels dry when you touch it.
4 Mix the soil in a basket and scatter a thin layer over newspaper to dry. Leave the soil to dry out until it feels dry when you touch it. - It is very important to make sure that the soil is completely dry before starting the pH measurement procedure. Soil moisture can falsify pH readings.
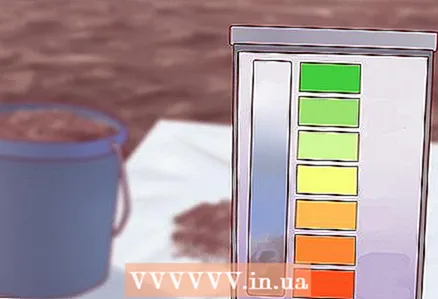
 5 Use the kit to determine the exact pH level of your soil. The determination methodology will depend on your specific test kit. For most kits, it is necessary to put a small amount of soil in a special test tube, add a few drops of a special solution to it, shake thoroughly and let the resulting suspension settle for several hours. After a certain time, the color of the solution should change, and by comparing the resulting solution with the color chart that came with the test, you can determine the pH of your soil.
5 Use the kit to determine the exact pH level of your soil. The determination methodology will depend on your specific test kit. For most kits, it is necessary to put a small amount of soil in a special test tube, add a few drops of a special solution to it, shake thoroughly and let the resulting suspension settle for several hours. After a certain time, the color of the solution should change, and by comparing the resulting solution with the color chart that came with the test, you can determine the pH of your soil. - There are other soil pH testing kits available, so follow the instructions that came with your kit. For example, some modern electronic pH meters measure the indicator almost instantly using a metal sample.
Part 2 of 3: Using Techniques to Lower the pH
 1 Add organic materials. Many organic materials such as compost, composted manure and acidic mulch (such as pine needles) can gradually lower soil pH over time. As organic materials decompose, bacteria and other microorganisms grow and feed on them, releasing acidic by-products. Since organic materials take a long time to decompose and change the soil, this method works well for long-term purposes. However, if you want quick results, this method will not live up to your expectations. Many gardeners prefer to add organic materials to the soil annually to slowly gradually lower the soil pH.
1 Add organic materials. Many organic materials such as compost, composted manure and acidic mulch (such as pine needles) can gradually lower soil pH over time. As organic materials decompose, bacteria and other microorganisms grow and feed on them, releasing acidic by-products. Since organic materials take a long time to decompose and change the soil, this method works well for long-term purposes. However, if you want quick results, this method will not live up to your expectations. Many gardeners prefer to add organic materials to the soil annually to slowly gradually lower the soil pH. - Organic fertilizers can provide additional benefits to the soil - the most famous of which are improved soil drainage and aeration.
 2 Add aluminum sulfate. It is not necessary to rely on the gradual, slow decomposition of the organic substrate to quickly lower the soil pH. On the contrary, in any gardening store you can find a wide variety of additives that quickly acidify the soil. Among these additives, you can choose aluminum sulfate - one of the fastest-acting substances. Aluminum sulfate releases acid into the soil as it dissolves, which in horticulture means it works almost instantly. Therefore, it is aluminum sulfate that will help you if you need to quickly lower the pH of the soil in your garden.
2 Add aluminum sulfate. It is not necessary to rely on the gradual, slow decomposition of the organic substrate to quickly lower the soil pH. On the contrary, in any gardening store you can find a wide variety of additives that quickly acidify the soil. Among these additives, you can choose aluminum sulfate - one of the fastest-acting substances. Aluminum sulfate releases acid into the soil as it dissolves, which in horticulture means it works almost instantly. Therefore, it is aluminum sulfate that will help you if you need to quickly lower the pH of the soil in your garden. - Depending on the starting pH of your soil, the amount of aluminum sulfate you will need to use varies greatly. By very As a rough estimate, you should calculate that in order to lower the pH by one unit (that is, from 7 to 6, from 6 to 5) on a plot of land measuring 1 square meter, you will need 550 grams of aluminum sulfate. However, if you add too much aluminum sulfate it can harm your plantings, so consult the relevant internet sites (like here) for more details.
 3 Add sulfur. Another substance that is added to soil to lower the pH is freeze-dried sulfur. If we compare this additive with aluminum sulfate, then it is somewhat cheaper, it is required less per unit area, but it acts somewhat slower. Since sulfur must be absorbed by soil bacteria, which then convert it to sulfuric acid, this process takes some time. Depending on soil moisture, bacteria and temperature, it can take up to several months for sulfur to begin to have a pronounced effect on soil acidity.
3 Add sulfur. Another substance that is added to soil to lower the pH is freeze-dried sulfur. If we compare this additive with aluminum sulfate, then it is somewhat cheaper, it is required less per unit area, but it acts somewhat slower. Since sulfur must be absorbed by soil bacteria, which then convert it to sulfuric acid, this process takes some time. Depending on soil moisture, bacteria and temperature, it can take up to several months for sulfur to begin to have a pronounced effect on soil acidity. - As noted above, unlike aluminum sulfate, you usually need a relatively small amount of pure, sublimated sulfur to achieve an equivalent pH change. On average, you will need 90 grams of sulfur to lower the pH by one unit on a 1 square meter plot of soil. Consult the dedicated website (eg here) for more detailed usage information.
 4 Add granular sulfur coated urea. Like aluminum sulfate and sulfur, soil additives containing sulfur-coated urea can gradually increase the acidity of the mail (lower its pH). Additives containing urea act quite quickly, and the effect begins to appear 1-2 weeks after the introduction of the substance into the soil. Sulfur-coated urea is a common ingredient in many fertilizers, so if you plan to feed your plants with fertilizer, you can avoid wasting time and money on this supplement and choose a fertilizer that contains this substance right away.
4 Add granular sulfur coated urea. Like aluminum sulfate and sulfur, soil additives containing sulfur-coated urea can gradually increase the acidity of the mail (lower its pH). Additives containing urea act quite quickly, and the effect begins to appear 1-2 weeks after the introduction of the substance into the soil. Sulfur-coated urea is a common ingredient in many fertilizers, so if you plan to feed your plants with fertilizer, you can avoid wasting time and money on this supplement and choose a fertilizer that contains this substance right away. - The content of sulfur-coated urea differs depending on the type of fertilizer chosen, so carefully read the fertilizer instructions to determine how much of the substance will be needed for the needs of your garden.
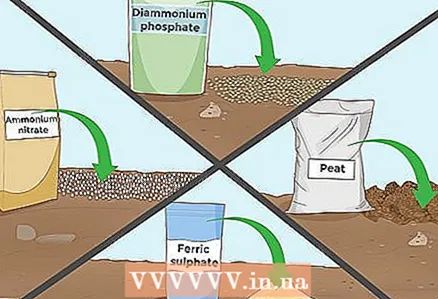 5 Add other acidic additives. In addition to the additives listed above, there are many other substances that are sold both separately and as part of complex fertilizers. The amount of fertilizer and when to apply it is very dependent on the type of fertilizer, so carefully read the instructions on the product packaging or ask a consultant at your gardening store for advice. Here are some additives that can lower the pH level of your soil:
5 Add other acidic additives. In addition to the additives listed above, there are many other substances that are sold both separately and as part of complex fertilizers. The amount of fertilizer and when to apply it is very dependent on the type of fertilizer, so carefully read the instructions on the product packaging or ask a consultant at your gardening store for advice. Here are some additives that can lower the pH level of your soil: - Ammonium hydrogen phosphate
- Copper sulphate
- Peat
- Ammonium nitrate
 6 Grow plants adapted to alkaline soils. If your soil is too alkaline to grow plants that need acidic soils, growing plants that prefer alkaline soils can significantly lower the pH for almost its entire life. As the plants grow, mature and die off, the organic substrate that enters the soil causes bacteria to develop, and the soil pH gradually decreases (the same principle applies here as when applying organic material in the form of mulch or manure). This method is one of the slowest ways to lower the pH, because the plants must first grow before they begin to supply organic material to the soil. Here are some examples of plants that prefer alkaline soil:
6 Grow plants adapted to alkaline soils. If your soil is too alkaline to grow plants that need acidic soils, growing plants that prefer alkaline soils can significantly lower the pH for almost its entire life. As the plants grow, mature and die off, the organic substrate that enters the soil causes bacteria to develop, and the soil pH gradually decreases (the same principle applies here as when applying organic material in the form of mulch or manure). This method is one of the slowest ways to lower the pH, because the plants must first grow before they begin to supply organic material to the soil. Here are some examples of plants that prefer alkaline soil: - Certain deciduous shrubs (such as lilacs, rose hips, clematis, and honeysuckle)
- Certain evergreen shrubs (such as boxwood)
- Certain perennials (such as chrysanthemums)
Part 3 of 3: When to Lower Soil pH
 1 Lower soil pH for shrubs such as rhododendron or azalea. Some types of flowering shrubs, such as rhododendron and azalea, require a fairly acidic soil to grow well. These plants originate from areas where there is a lot of rainfall (such as the Pacific Northwest region of the United States), and high rainfall contributes to the acidification of the soil. For these plant species, the optimum pH value ranges from 4.5 to 5.5. However, they can grow in soils with a pH of 6.0.
1 Lower soil pH for shrubs such as rhododendron or azalea. Some types of flowering shrubs, such as rhododendron and azalea, require a fairly acidic soil to grow well. These plants originate from areas where there is a lot of rainfall (such as the Pacific Northwest region of the United States), and high rainfall contributes to the acidification of the soil. For these plant species, the optimum pH value ranges from 4.5 to 5.5. However, they can grow in soils with a pH of 6.0. 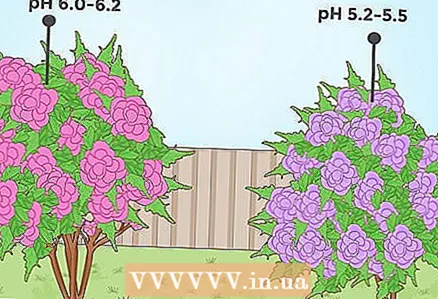 2 Lower the pH for flowers such as petunia or begonia. Many brightly flowering plants, such as petunias and begonia, do better in acidic soils. For some of these colors, acidity changes from slightly acidic before very acidic can lead to a visible discoloration of the flowers. For example, if you are growing a hydrangea in an area where the soil pH is 6.0-6.2, then pink flowers will bloom on the plant. If you lower the pH to 5.0-5.2, then you will have flowers with blue or purple petals.
2 Lower the pH for flowers such as petunia or begonia. Many brightly flowering plants, such as petunias and begonia, do better in acidic soils. For some of these colors, acidity changes from slightly acidic before very acidic can lead to a visible discoloration of the flowers. For example, if you are growing a hydrangea in an area where the soil pH is 6.0-6.2, then pink flowers will bloom on the plant. If you lower the pH to 5.0-5.2, then you will have flowers with blue or purple petals. - The blue color of the petals of hydrangea grown at low soil pH is due to aluminum salts. When the soil is acidic, the plant absorbs aluminum from the substrate much more easily, which affects the color of its petals.
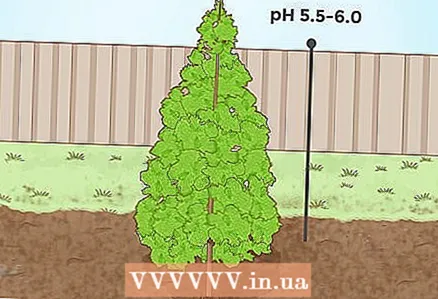 3 Lower the pH level for evergreen trees. Many evergreen conifers grow on slightly acidic soils. For example, spruce, pine and fir thrive if the soil pH is 5.5-6.0. In addition, the needles of these tree species can be applied as organic material to alkaline and neutral soils. As the needles decompose, the pH level will slowly decrease.
3 Lower the pH level for evergreen trees. Many evergreen conifers grow on slightly acidic soils. For example, spruce, pine and fir thrive if the soil pH is 5.5-6.0. In addition, the needles of these tree species can be applied as organic material to alkaline and neutral soils. As the needles decompose, the pH level will slowly decrease. 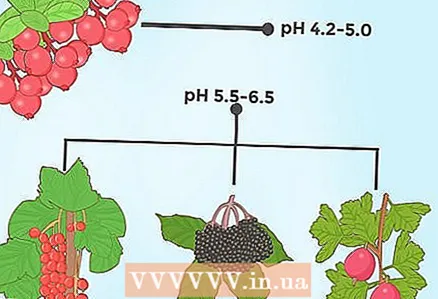 4 Lower soil pH for certain berry crops. Probably the most famous berry plant that requires acidic soil is blueberry, which grows well in very acidic soils (ideal pH values are 4.0-5.0). There are other berries that prefer acidic soils. So, for example, cranberries grow well at a pH of 4.2-5.0, and cloudberries, currants and elderberries - at a pH of 5.05-6.5.
4 Lower soil pH for certain berry crops. Probably the most famous berry plant that requires acidic soil is blueberry, which grows well in very acidic soils (ideal pH values are 4.0-5.0). There are other berries that prefer acidic soils. So, for example, cranberries grow well at a pH of 4.2-5.0, and cloudberries, currants and elderberries - at a pH of 5.05-6.5. 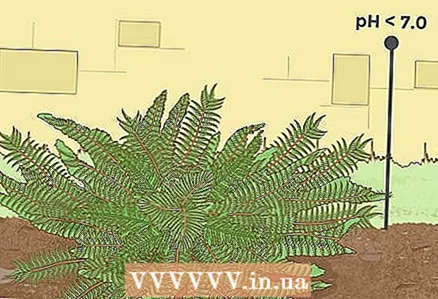 5 For ferns, you need to lower the acidity of the soil only slightly below neutral. Most garden fern varieties prefer soils where the pH is slightly below 7.0. Even those that prefer alkaline soils can tolerate slightly acidic substrates. For example, a maidenhair that prefers soils with a pH of 7.0-8.0 can do pretty well at a pH of 6.0. Some ferns can even tolerate acidic soils with a pH of 4.0.
5 For ferns, you need to lower the acidity of the soil only slightly below neutral. Most garden fern varieties prefer soils where the pH is slightly below 7.0. Even those that prefer alkaline soils can tolerate slightly acidic substrates. For example, a maidenhair that prefers soils with a pH of 7.0-8.0 can do pretty well at a pH of 6.0. Some ferns can even tolerate acidic soils with a pH of 4.0.  6 Find specific sources of information for gardeners and gardeners for a detailed list of plants that prefer acidic soil. The list of plants that can or prefer to grow in acidic soils is too extensive to be included in this article. For more complete information, you can refer to special botanical reference books. They can usually be found in gardening stores or purchased from a special section of any bookstore. Alternatively, you can find information on the Internet. For example, the official website of the "Old Farmer's Almanac" magazine contains a table that shows the preference for soil acidity for many plants (you can find it here).
6 Find specific sources of information for gardeners and gardeners for a detailed list of plants that prefer acidic soil. The list of plants that can or prefer to grow in acidic soils is too extensive to be included in this article. For more complete information, you can refer to special botanical reference books. They can usually be found in gardening stores or purchased from a special section of any bookstore. Alternatively, you can find information on the Internet. For example, the official website of the "Old Farmer's Almanac" magazine contains a table that shows the preference for soil acidity for many plants (you can find it here).
Tips
- Some additives that alter the acidity of the soil are sold as sprays.
- It is very important not to overdo it with the amount of soil additives used. They have a long-term effect on the soil and on the environment in general.
- Plants grown in soil with an inappropriate pH for them cannot grow well because some of the nutrients may be bound in the soil and thus not available to plants.
- The effect after the introduction of natural sulfur will persist for several seasons.
- It is best to apply sulfur in early spring, when the plants are already planted, it is very difficult to add sulfur to the soil.
- Soil pH is influenced by many factors, from how well the site is drained to how quickly the erosion process occurs.
- Use natural compost if possible. It is very beneficial for plants because it provides them with many nutrients. You can even compost from kitchen waste and grass cut from the lawn.
- Sulfur and compost create conditions for biochemical reactions in the soil, while aluminum and iron sulfates undergo chemical interaction.
Warnings
- Too much aluminum sulfate can poison the soil.
- If you sprayed urea, aluminum sulfate, or sulfur and it gets on the plant leaves, rinse it off with plenty of cool water. If you leave these chemicals on the leaves, they will "burn" the leaves, causing unsightly stains.