Author:
John Stephens
Date Of Creation:
27 January 2021
Update Date:
1 July 2024

Content
In chemistry, electronegativity is the unit for measuring the attraction of an atom to the electron in chemical bond. Atoms with high electronegativity will attract electrons with strong force, whereas atoms with low electronegativity will attract electrons with weak force. Electronegativity values are used to predict the ability to form chemical bonds between atoms, so this is an important skill in basic chemistry.
Steps
Method 1 of 3: Basic knowledge of electronegativity
Chemical bonding arises when atoms share electrons. To understand electronegativity, you must first understand what "bonding" is. Any two atoms that are "connected" together in the molecular structure will have a bond between them, meaning that they share a pair of electrons and each atom contributes one electron to that bond.
- This article does not cover the exact reason why atoms share electrons and have a bond between them. If you want to learn more, read this article on chemical bonding or wikiHow's article on How to study chemical bond properties.

How does electronegativity affect electrons in the bond? When two atoms share the same electron pair in bond, this share is not always in equilibrium. When one atom has a higher electronegativity than the other, it pulls the two electrons in the bond closer to it. An atom has a very high electronegativity that can pull electrons towards it almost completely, and hardly share electrons with the other atom.- For example, in the NaCl (sodium chloride) molecule, the chlorine atom has a relatively high electronegativity and the sodium atom has a relatively low electronegativity. Hence the electrons are pulled towards the chlorine atom and away from sodium atoms.

Use the electronegativity table for reference. On the electronegativity table, the chemical elements are arranged exactly as in the periodic table, but electronegativity is recorded on each atom. This chart is printed in many chemistry textbooks, technical literature, or on the internet.- This is the connection that leads to the electronegativity checker. Note that this table uses the Pauling scale, which is the most common electronegativity scale. However, there are other ways to measure electronegativity, and one of them will be outlined below.

The atoms are arranged in electronegativity for easy estimation. If you don't have an electronegativity chart, you can estimate an atom's electronegativity based on its position on a regular chemical periodic table. As a general rule:- Electronegativity of the atom gradually higher when you move on the right periodic table.
- Electronegativity of the atom gradually higher as you move go up periodic table.
- Therefore, the atoms in the upper right corner have the highest electronegativity, and the atoms in the lower left corner have the lowest electronegativity.
- In the NaCl example above, you can tell that chlorine has a higher electronegativity than sodium because it is very close to the top right corner of the periodic table. In contrast, sodium is far to the left so it belongs to the group of atoms with low electronegativity.
Method 2 of 3: Determine the bond type by electronegativity
Find out the electronegativity difference between two atoms. When two atoms are bonded, the difference in electronegativity between the two atoms can tell you the properties of that bond. Subtract the small electronegativity from the small electronegativity to find the difference.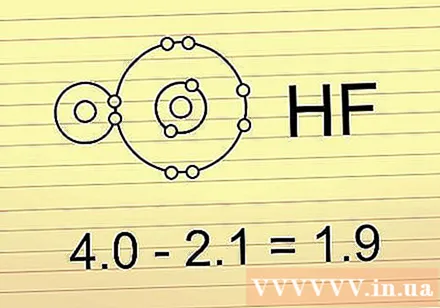
- Taking the HF molecule as an example, we will subtract the electronegativity of fluorine (4,0) for the electronegativity of hydrogen (2,1). 4.0 - 2.1 = 1,9.
If the electronegativity difference is less than about 0.5 then the bond is a nonpolar covalent bond, in which electrons are shared almost equally. This type of bond does not create a molecule with a large difference in charge between the ends of the bond. Non-polar bonds are often difficult to break.
- For example, molecule O2 have this type of link. Since the two oxygen atoms have the same electronegativity, their difference is zero.
If the electronegativity difference is between 0.5-1.6 then the bond is a polar covalent bond. These bonds have more electrons on one end than the other. This causes the molecule to have a slightly larger negative charge on the end of the electron, and a slightly larger net of positive charge on the other end. The charge imbalance in the bond allows the molecule to participate in a number of special reactions.
- Molecular H2O (water) is a prime example of this. The O atom has a greater electronegativity than two H atoms, so it holds electrons tighter, and causes the entire molecule to carry some negative charge at the O end and part positively on the H end.
If the electronegativity difference is greater than 2.0 then the bond is an ionic bond. In this bond, electrons are located entirely at one end of the bond. Atoms with a greater electronegativity have a negative charge, and atoms with a smaller electronegativity have a positive charge. This type of bonding allows the atom in it to react well with other atoms, and even be separated by polar atoms.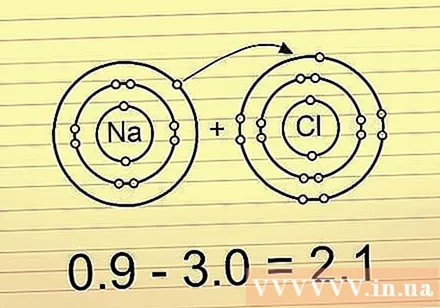
- An example is the BaCl molecule (sodium chloride). The chlorine atom has such a large negative charge that it pulls both electrons completely toward it, causing sodium to be positively charged.
If the electronegativity difference is between 1.6-2.0, check metallic element. If have a metal element in the bond is the bond ions. If there are no metallic elements, it is bonding polar covalent.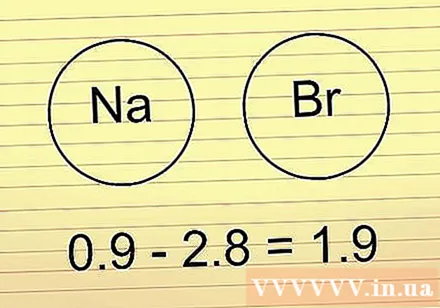
- Metallic elements include most of the elements on the left and middle of the periodic table. This page has a table showing which elements are metallic.
- The above HF example is in this range. Since H and F are not metals, they are bonded polar covalent.
Method 3 of 3: Find the electronegativity according to Mulliken
Find the first ionizing energy of the atom. Electronegativity according to Mulliken is a method of measuring electronegativity slightly different from the Pauling scale method mentioned above. To find Mulliken electronegativity for a given atom, find its first ionizing energy. This is the energy required for the atom to give away an electron.
- You may have to look this up in your chemical references. This page provides a lookup table that you can use (scroll down to see).
- For example, suppose we need to find the electronegativity of lithium (Li). Looking at the table on the above page, we see that the first ionization energy is 520 kJ / mol.
Find the electronic affinity of the atom. This is a measure of the energy obtained when an atom receives an electron to form a negative ion. You must also look up this parameter in your chemical references. This site has learning resources you should be looking for.
- Lithium's electronic affinity is 60 kJ mol.
Solve equations of electronegativity according to Mulliken. When you use kJ / mol for energy, the electronegativity equation according to Mulliken is ENMulliken = (1.97 × 10) (Ei+ Eea) + 0,19. Plug the values into the equation and solve for ENMulliken.
- In this example, we will solve the following:
- ENMulliken = (1.97 × 10) (Ei+ Eea) + 0,19
- ENMulliken = (1,97×10)(520 + 60) + 0,19
- ENMulliken = 1,143 + 0,19 = 1,333
- In this example, we will solve the following:
Advice
- In addition to the Pauling and Mulliken scales, some other electronegativity scales are Allred – Rochow, Sanderson and Allen. All of these scales have their own equations for calculating electronegativity (a fairly complicated number).
- Electronegativity no unit.