Author:
Florence Bailey
Date Of Creation:
21 March 2021
Update Date:
1 July 2024
Content
- Steps
- Method 1 of 3: Fast translation for culinary ingredients
- Method 2 of 3: Breaking down the basics
- Method 3 of 3: Calculate the translation formula yourself
- Tips
- Warnings
Converting milliliters (ml) to grams (g) is more difficult than just adding zeros to the value, since you need to convert units of volume - millimeters - to mass units - grams. This means that each substance will have its own formula for transformation, but all of them will not require knowledge of mathematics more difficult than multiplication. Such transformations are commonly used to translate recipes from one measurement system to another, or to solve chemical problems.
Steps
Method 1 of 3: Fast translation for culinary ingredients
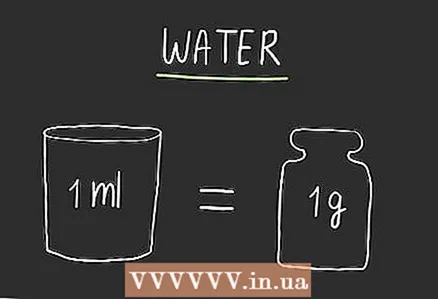 1 Do nothing to convert the volume of water. One milliliter of water has a mass of one gram and in normal situations, including recipes and math and scientific problems (unless otherwise specified). There is no need to resort to calculations: the values in millimeters and grams are always the same.
1 Do nothing to convert the volume of water. One milliliter of water has a mass of one gram and in normal situations, including recipes and math and scientific problems (unless otherwise specified). There is no need to resort to calculations: the values in millimeters and grams are always the same. - Such a simple transformation is not a coincidence, but a result of how these measures are defined. Many scientific units of measurement were determined using water, because water is a common and useful substance.
- The only time you will need to use a different formula is if the water turns out to be extremely hot or colder than possible in everyday life.
 2 To convert for milk, multiply by 1.03. Multiply the ml value for milk by 1.03 to get the mass (or weight) in grams. This formula is suitable for fatty milk. For fat-free, the ratio is closer to 1.035, but this is not important for most recipes.
2 To convert for milk, multiply by 1.03. Multiply the ml value for milk by 1.03 to get the mass (or weight) in grams. This formula is suitable for fatty milk. For fat-free, the ratio is closer to 1.035, but this is not important for most recipes. 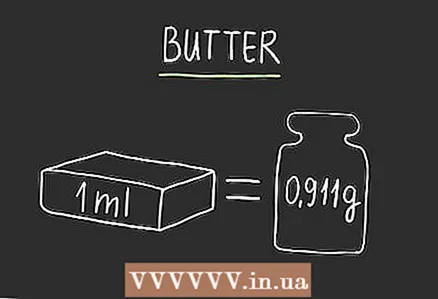 3 To convert for oil, multiply by 0.911. If you don't have a calculator, 0.9 is sufficient for most recipes.
3 To convert for oil, multiply by 0.911. If you don't have a calculator, 0.9 is sufficient for most recipes. 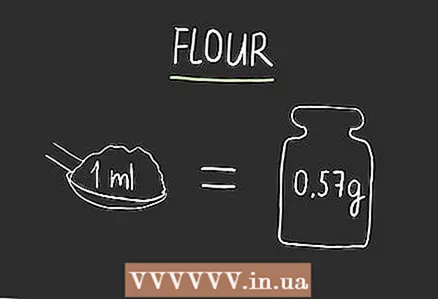 4 To convert for flour, multiply by 0.57. There are many different types of flour, but most varieties - whether it's general purpose flour, whole grain flour, or bread flour - have roughly the same gravity. Since there are many varieties, add a little flour to the dish, using more or less depending on how the dough or mixture looks.
4 To convert for flour, multiply by 0.57. There are many different types of flour, but most varieties - whether it's general purpose flour, whole grain flour, or bread flour - have roughly the same gravity. Since there are many varieties, add a little flour to the dish, using more or less depending on how the dough or mixture looks. - These measurements were carried out at a density of 8.5 grams per tablespoon, and the volume of one tablespoon is 14.7868 ml.
 5 Use the online ingredient calculator. Most types of products are included in this calculator. A milliliter is the same as a cubic centimeter, so select the cubic centimeter option, enter the volume in milliliters, and then the type of product or ingredient you want to find by weight.
5 Use the online ingredient calculator. Most types of products are included in this calculator. A milliliter is the same as a cubic centimeter, so select the cubic centimeter option, enter the volume in milliliters, and then the type of product or ingredient you want to find by weight.
Method 2 of 3: Breaking down the basics
 1 Understand milliliters and volume. Milliliters - units volume, or occupied space. One milliliter of water, one milliliter of gold, one milliliter of air will occupy the same space. If you break an item to make it smaller and denser it is will change its volume. Approximately twenty drops of water, or 1/5 teaspoon, takes up one milliliter.
1 Understand milliliters and volume. Milliliters - units volume, or occupied space. One milliliter of water, one milliliter of gold, one milliliter of air will occupy the same space. If you break an item to make it smaller and denser it is will change its volume. Approximately twenty drops of water, or 1/5 teaspoon, takes up one milliliter. - The milliliter is reduced to ml.
 2 Understand grams and weight. Gram - unit masses or the amount of the substance. If you break an item to make it smaller and denser it is will not change its mass. A paper clip, bag of sugar, or a zest weighs one gram each.
2 Understand grams and weight. Gram - unit masses or the amount of the substance. If you break an item to make it smaller and denser it is will not change its mass. A paper clip, bag of sugar, or a zest weighs one gram each. - The gram is often used as a unit of weight and can be measured using a scale in everyday situations. Weight is the value of the force of gravity acting on a mass. If you went into space, you would still have the same mass (amount of matter), but you would not have weight, since there is no gravity there.
- The gram is reduced to G.
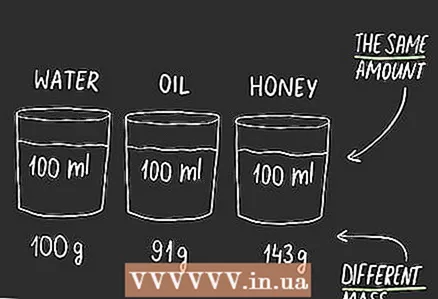 3 Understand why you need to know for which substance you are translating the meaning. Since units measure different things, there is no formula for quick translation between them. You will need to find the formula depending on the object to be measured. For example, molasses in a milliliter container will not have the same weight as water in a container of the same volume.
3 Understand why you need to know for which substance you are translating the meaning. Since units measure different things, there is no formula for quick translation between them. You will need to find the formula depending on the object to be measured. For example, molasses in a milliliter container will not have the same weight as water in a container of the same volume. 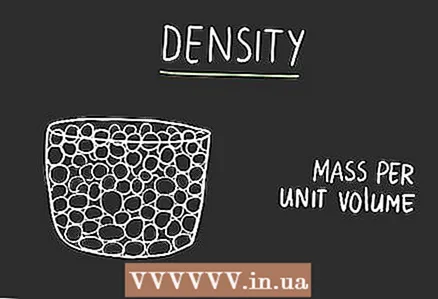 4 Get to know density. Density indicates how strongly the substance in an object is grouped together.We can distinguish density in everyday life without even measuring it. If you pick up a metal ball, you will be surprised how much it weighs for its size. This will happen due to the fact that it has a high density. A large amount of matter is grouped in a small space. If you pick up a crumpled ball of the same size, you can easily throw it. The paper ball has a low density. Density is measured in units of mass per unit volume. For example, how much masses in grams fits in one milliliter volume... Therefore, it can be used to convert between two units of measurement.
4 Get to know density. Density indicates how strongly the substance in an object is grouped together.We can distinguish density in everyday life without even measuring it. If you pick up a metal ball, you will be surprised how much it weighs for its size. This will happen due to the fact that it has a high density. A large amount of matter is grouped in a small space. If you pick up a crumpled ball of the same size, you can easily throw it. The paper ball has a low density. Density is measured in units of mass per unit volume. For example, how much masses in grams fits in one milliliter volume... Therefore, it can be used to convert between two units of measurement.
Method 3 of 3: Calculate the translation formula yourself
 1 Try to find the density of the substance. As described above, density is the ratio of mass to unit volume. If you are solving a problem in chemistry or math, this can help you find out the density of a substance. Otherwise, look for the density of the substance online or in a table.
1 Try to find the density of the substance. As described above, density is the ratio of mass to unit volume. If you are solving a problem in chemistry or math, this can help you find out the density of a substance. Otherwise, look for the density of the substance online or in a table. - Use this table to view the density of any pure element. (note that 1 cm = 1 milliliter).
- Use this document to find out the gravity for many foods and drinks. For items that have a "specific gravity" value, this number will be equal to the density in g / ml at 4ºC (39ºF), and it will be quite close to the density of the substance at room temperature.
- For other substances, enter the name and the word "density" in the search engine.
 2 Convert density to g / ml if necessary. Sometimes the density is given in units other than g / ml. If the density is written in g / cm, no changes need to be made, since cm is just 1 ml. For other units, try using the online density conversion calculator or do the calculations yourself:
2 Convert density to g / ml if necessary. Sometimes the density is given in units other than g / ml. If the density is written in g / cm, no changes need to be made, since cm is just 1 ml. For other units, try using the online density conversion calculator or do the calculations yourself: - Multiply the density in kg / m3 (kilogram per cubic meter) by 0.001 to get the density in g / ml.
- Multiply the density in lb / gallon (pound per US gallon) by 0.120 to get the density in g / ml.
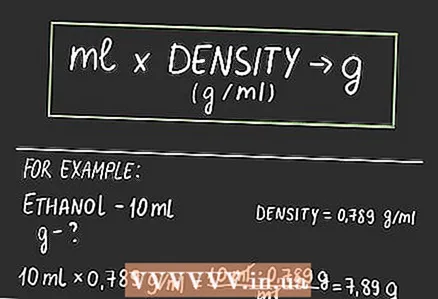 3 Multiply the volume in milliliters by the density. Multiply the volume of your substance in ml by its density in g / ml. The answer will be in (g x ml) / ml. But you can subtract ml at the top and bottom of the fraction, and you are left with g or grams.
3 Multiply the volume in milliliters by the density. Multiply the volume of your substance in ml by its density in g / ml. The answer will be in (g x ml) / ml. But you can subtract ml at the top and bottom of the fraction, and you are left with g or grams. - For example, let's convert 10 ml of ethanol to grams. Find the density of ethanol: 0.789 g / ml. Multiply 10 ml by 0.789 g / ml to get 7.89 grams. We now know that 10 ml of ethanol weighs 7.89 grams.
Tips
- To convert grams to milliliters, divide grams by density instead of multiplying.
- The density of water is 1 g / ml. If the density of a substance is greater than 1 g / ml, then it is denser than pure water and will sink to the bottom. If the density of a substance is less than 1 g / ml, then it will float up, since it is less dense than water.
Warnings
- Objects can expand and shrink if you change the temperature, especially if they melt, freeze, and the like. However, if the state of the substance is known (such as solid or liquid) and you are working under normal day-to-day conditions, you can use the “normal” density.