Author:
Janice Evans
Date Of Creation:
25 July 2021
Update Date:
1 July 2024

Content
Ionic equations are an integral part of chemistry. They contain only those components that change in the course of a chemical reaction. Most often, ionic equations are used to describe redox reactions, exchange and neutralization reactions.Writing an ionic equation requires three basic steps: balancing the molecular equation of a chemical reaction, translating it into a complete ionic equation (that is, writing the components as they exist in solution), and finally writing a short ionic equation.
Steps
Part 1 of 2: Components of the Ionic Equation
 1 Understand the difference between molecular and ionic compounds. To write the ionic equation, the first step is to determine the ionic compounds involved in the reaction. Ionic are those substances that dissociate (decompose) into charged ions in aqueous solutions. Molecular compounds do not break down into ions. They are composed of two non-metallic elements and are sometimes referred to as covalent compounds.
1 Understand the difference between molecular and ionic compounds. To write the ionic equation, the first step is to determine the ionic compounds involved in the reaction. Ionic are those substances that dissociate (decompose) into charged ions in aqueous solutions. Molecular compounds do not break down into ions. They are composed of two non-metallic elements and are sometimes referred to as covalent compounds. - Ionic compounds can occur between a metal and a non-metal, a metal and polyatomic ions, or between several polyatomic ions.
- If you are in doubt as to which group a particular compound belongs to, look at the properties of its constituent elements in the periodic table.
 2 Determine the solubility of the compound. Not all ionic compounds dissolve in aqueous solutions, that is, not all of them dissociate into separate ions. Before you start writing the equation, you should find the solubility of each compound. Below are brief rules for solubility. More details and exceptions to the rule can be found in the dissolution table.
2 Determine the solubility of the compound. Not all ionic compounds dissolve in aqueous solutions, that is, not all of them dissociate into separate ions. Before you start writing the equation, you should find the solubility of each compound. Below are brief rules for solubility. More details and exceptions to the rule can be found in the dissolution table. - Follow the rules in the order in which they are given below:
- all salts Na, K and NH4 dissolve;
- all salts NO3, C2H3O2, ClO3 and ClO4 soluble;
- all salts Ag, Pb and Hg2 insoluble;
- all Cl, Br and I salts dissolve;
- salts CO3, O, S, OH, PO4, CrO4, Cr2O7 and SO3 insoluble (with some exceptions);
- SO salts4 soluble (with some exceptions).
 3 Determine the cation and anion of the compound. Positively charged ions (usually metals) are called cations. Anions have a negative charge, usually non-metal ions. Some non-metals can form not only anions, but also cations, while metal atoms always act as cations.
3 Determine the cation and anion of the compound. Positively charged ions (usually metals) are called cations. Anions have a negative charge, usually non-metal ions. Some non-metals can form not only anions, but also cations, while metal atoms always act as cations. - For example, in the compound NaCl (table salt), Na is a positively charged cation, since it is a metal, and Cl is a negatively charged anion, since it is a non-metal.
 4 Determine the polyatomic (complex) ions involved in the reaction. Such ions are charged molecules, between whose atoms there is such a strong bond that they do not dissociate in chemical reactions. It is necessary to identify polyatomic ions, since they have their own charge and do not decay into individual atoms. Polyatomic ions can have both positive and negative charges.
4 Determine the polyatomic (complex) ions involved in the reaction. Such ions are charged molecules, between whose atoms there is such a strong bond that they do not dissociate in chemical reactions. It is necessary to identify polyatomic ions, since they have their own charge and do not decay into individual atoms. Polyatomic ions can have both positive and negative charges. - In your general chemistry course, you will likely need to memorize some of the most common polyatomic ions.
- The most common polyatomic ions are CO3, NO3, NO2, SO4, SO3, ClO4 and ClO3.
- There are many other polyatomic ions that can be found in a chemistry textbook or on the internet.
Part 2 of 2: Writing Ionic Equations
 1 Balance the complete molecular equation. Before you start writing the ionic equation, you need to balance the original molecular equation. To do this, it is necessary to place the corresponding coefficients in front of the compounds, so that the number of atoms of each element on the left side is equal to their number on the right side of the equation.
1 Balance the complete molecular equation. Before you start writing the ionic equation, you need to balance the original molecular equation. To do this, it is necessary to place the corresponding coefficients in front of the compounds, so that the number of atoms of each element on the left side is equal to their number on the right side of the equation. - Write down the number of atoms for each element on either side of the equation.
- Add coefficients before the elements (except oxygen and hydrogen) so that the number of atoms of each element on the left and right sides of the equation is the same.
- Balance the hydrogen atoms.
- Balance the oxygen atoms.
- Count the number of atoms for each element on either side of the equation and make sure it is the same.
- For example, after balancing the Cr + NiCl equation2 -> CrCl3 + Ni we get 2Cr + 3NiCl2 -> 2CrCl3 + 3Ni.
 2 Determine the state of each substance that participates in the reaction. This can often be judged by the condition of the problem. There are certain rules that help determine what state an element or a connection is in.
2 Determine the state of each substance that participates in the reaction. This can often be judged by the condition of the problem. There are certain rules that help determine what state an element or a connection is in. - If the state of an element is not indicated in the condition of the problem, use the periodic table to determine it.
- If the condition says the compound is in solution, mark it (rr).
- If water is included in the equation, use the solubility table to determine if the ionic compound will dissociate. In the case of high solubility, the compound dissociates in water (rr). If the compound has low solubility, it will remain solid (tv).
- If water does not participate in the reaction, the ionic compound will remain in solid form (tv).
- If an acid or a base appears in the problem, they will be dissolved in water (rr).
- As an example, consider the reaction 2Cr + 3NiCl2 -> 2CrCl3 + 3Ni. In pure form, the elements Cr and Ni are in the solid phase. NiCl2 and CrCl3 are soluble ionic compounds, that is, they are in solution. Thus, this equation can be rewritten as follows: 2Cr(tv) + 3NiCl2(rr) -> 2CrCl3(rr) + 3Ni(tv).
 3 Determine which compounds dissociate (separate into cations and anions) in solution. Upon dissociation, the compound decomposes into positive (cation) and negative (anion) components. These components will then enter the ionic equation of the chemical reaction.
3 Determine which compounds dissociate (separate into cations and anions) in solution. Upon dissociation, the compound decomposes into positive (cation) and negative (anion) components. These components will then enter the ionic equation of the chemical reaction. - Solids, liquids, gases, molecular compounds, ionic compounds with low solubility, polyatomic ions and weak acids do not dissociate.
- Fully dissociates highly soluble ionic compounds (use the solubility table) and strong acids (HCl(rr), HBr(rr), HI(rr), H2SO4(rr), HClO4(rr) and HNO3(rr)).
- Note that although polyatomic ions do not dissociate, they can be incorporated into the ionic compound and separated from it in solution.
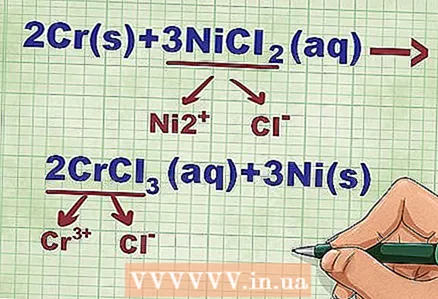 4 Calculate the charge of each dissociated ion. In doing so, remember that metals form positively charged cations, and non-metal atoms turn into negative anions. Determine the charges of the elements according to the periodic table. It is also necessary to balance all charges in neutral compounds.
4 Calculate the charge of each dissociated ion. In doing so, remember that metals form positively charged cations, and non-metal atoms turn into negative anions. Determine the charges of the elements according to the periodic table. It is also necessary to balance all charges in neutral compounds. - In the above example, NiCl2 dissociates into Ni and Cl, and CrCl3 decomposes into Cr and Cl.
- The nickel ion has a 2+ charge because it is bonded to two chlorine ions, each with a single negative charge. In this case, one Ni ion must balance two negatively charged Cl ions. The Cr ion has a 3+ charge, since it must neutralize three negatively charged Cl ions.
- Remember that polyatomic ions have their own charges.
 5 Rewrite the equation so that all soluble compounds are separated into individual ions. Anything that dissociates or ionizes (like strong acids) breaks down into two separate ions. In this case, the substance will remain in a dissolved state (rr). Check that the equation is balanced.
5 Rewrite the equation so that all soluble compounds are separated into individual ions. Anything that dissociates or ionizes (like strong acids) breaks down into two separate ions. In this case, the substance will remain in a dissolved state (rr). Check that the equation is balanced. - Solids, liquids, gases, weak acids and ionic compounds with low solubility will not change their state and will not separate into ions. Leave them as they were.
- Molecular compounds will simply scatter in solution, and their state will change to dissolved (rr). There are three molecular compounds that not will go to the state (rr), this is CH4(G), C3H8(G) and C8H18(f).
- For the reaction under consideration, the complete ionic equation can be written in the following form: 2Cr(tv) + 3Ni(rr) + 6Cl(rr) -> 2Cr(rr) + 6Cl(rr) + 3Ni(tv)... If chlorine is not part of the compound, it breaks down into individual atoms, so we multiplied the number of Cl ions by 6 on both sides of the equation.
 6 Cancel the equal ions on the left and right sides of the equation. You can only cross out those ions that are completely identical on both sides of the equation (have the same charges, subscripts, and so on). Rewrite the equation without these ions.
6 Cancel the equal ions on the left and right sides of the equation. You can only cross out those ions that are completely identical on both sides of the equation (have the same charges, subscripts, and so on). Rewrite the equation without these ions. - In our example, both sides of the equation contain 6 Cl ions that can be crossed out. Thus, we get a short ionic equation: 2Cr(tv) + 3Ni(rr) -> 2Cr(rr) + 3Ni(tv).
- Check the result. The total charges of the left and right sides of the ionic equation must be equal.
Tips
- Train yourself always write down the state of aggregation of all components in all equations of chemical reactions.