Author:
Helen Garcia
Date Of Creation:
21 April 2021
Update Date:
1 July 2024

Content
A chemical equation is a symbolic representation of a chemical reaction. In this case, the reacting compounds (reagents) are written on the left, and the resulting substances (reaction products) - on the right side of the equation. An arrow is placed between them from left to right, which indicates the direction of the reaction. According to the law of conservation of mass, in the course of a chemical reaction, new atoms cannot appear or old ones disappear; therefore, the number of atoms in the reactants must be equal to the number of atoms in the products of the chemical reaction. This article describes how to balance chemical equations using different methods.
Steps
Method 1 of 2: Traditional Method
 1 Write down a chemical equation. As an example, consider the following reaction:
1 Write down a chemical equation. As an example, consider the following reaction: - C3H8 + O2 -> H2O + CO2
- This reaction describes the combustion of propane (C3H8) in the presence of oxygen to form water and carbon dioxide (carbon dioxide).
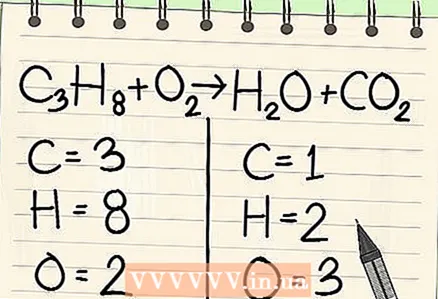 2 Write down the number of atoms for each element. Do this for both sides of the equation. Note the subscripts next to each element to determine the total number of atoms. Write down the symbol for each element in the equation and note the corresponding number of atoms.
2 Write down the number of atoms for each element. Do this for both sides of the equation. Note the subscripts next to each element to determine the total number of atoms. Write down the symbol for each element in the equation and note the corresponding number of atoms. - For example, on the right side of the equation under consideration, as a result of addition, we get 3 oxygen atoms.
- On the left side, we have 3 carbon atoms (C3), 8 hydrogen atoms (H8) and 2 oxygen atoms (O2).
- On the right side we have 1 carbon atom (C), 2 hydrogen atoms (H2) and 3 oxygen atoms (O + O2).
 3 Save hydrogen and oxygen for later, as they are part of several compounds on the left and right sides. Hydrogen and oxygen are part of several molecules, so it is best to balance them last.
3 Save hydrogen and oxygen for later, as they are part of several compounds on the left and right sides. Hydrogen and oxygen are part of several molecules, so it is best to balance them last. - Before balancing hydrogen and oxygen, you will have to recount the atoms again, as additional factors may be needed to balance other elements.
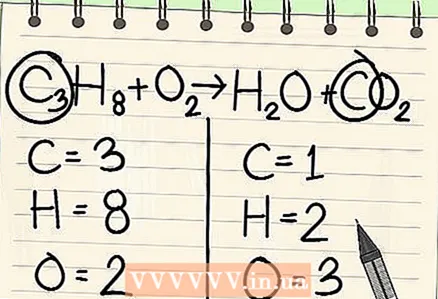 4 Start with the least frequent item. If you need to balance several elements, choose one that is part of one reagent molecule and one molecule of reaction products. So, the carbon must be balanced first.
4 Start with the least frequent item. If you need to balance several elements, choose one that is part of one reagent molecule and one molecule of reaction products. So, the carbon must be balanced first. 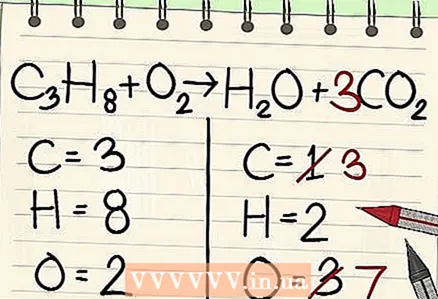 5 For balance, add a factor in front of a single carbon atom. Put a factor in front of the single carbon on the right side of the equation to balance it with the 3 carbons on the left side.
5 For balance, add a factor in front of a single carbon atom. Put a factor in front of the single carbon on the right side of the equation to balance it with the 3 carbons on the left side. - C3H8 + O2 -> H2O + 3CO2
- A factor of 3 in front of the carbon on the right side of the equation indicates that there are three carbon atoms, which correspond to the three carbon atoms in the propane molecule on the left side.
- In a chemical equation, you can change the coefficients in front of atoms and molecules, but the subscripts must remain unchanged.
 6 Then balance the hydrogen atoms. After you equalized the number of carbon atoms on the left and right sides, hydrogen and oxygen remained unbalanced. The left side of the equation contains 8 hydrogen atoms, the same number should be on the right. Achieve this with a ratio.
6 Then balance the hydrogen atoms. After you equalized the number of carbon atoms on the left and right sides, hydrogen and oxygen remained unbalanced. The left side of the equation contains 8 hydrogen atoms, the same number should be on the right. Achieve this with a ratio. - C3H8 + O2 -> 4H2O + 3CO2
- We've added a factor of 4 to the right, as the subscript shows that we already have two hydrogen atoms.
- If you multiply the factor 4 by the subscript 2, you get 8.
- As a result, 10 oxygen atoms are obtained on the right side: 3x2 = 6 atoms in three 3CO molecules2 and four more atoms in four water molecules.
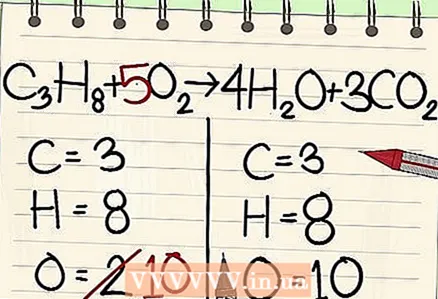 7 Balance the oxygen atoms. Remember to factor in the coefficients you used to balance the other atoms. Because you added the coefficients in front of the molecules on the right side of the equation, the number of oxygen atoms changed. You now have 4 oxygen atoms in water molecules and 6 oxygen atoms in carbon dioxide molecules. Thus, there are 10 oxygen atoms in the right side.
7 Balance the oxygen atoms. Remember to factor in the coefficients you used to balance the other atoms. Because you added the coefficients in front of the molecules on the right side of the equation, the number of oxygen atoms changed. You now have 4 oxygen atoms in water molecules and 6 oxygen atoms in carbon dioxide molecules. Thus, there are 10 oxygen atoms in the right side. - Add a factor of 5 to the oxygen molecule on the left side of the equation. Each piece now contains 10 oxygen atoms.
- C3H8 + 5O2 -> 4H2O + 3CO2.

- So, both sides of the equation contain the same number of carbon, hydrogen, and oxygen atoms. The equation is balanced.
Method 2 of 2: Algebraic Method
- 1 Write down the reaction equation. As an example, consider the following chemical reaction:
- PCl5 + H2O -> H3PO4 + HCl
- 2 Put a letter in front of each connection:
- aPCl5 + bH2O -> cH3PO4 + dHCl
- 3 Equate the number of atoms of each element on the left and right sides of the equation.
- aPCl5 + bH2O -> cH3PO4 + dHCl
- On the left we have 2b hydrogen atoms (2 in each H2O), while on the right is 3c+d hydrogen atoms (3 in each H3PO4 and 1 in each HCl molecule). Since the left and right sides must contain the same number of hydrogen atoms, 2b should be equal to 3c+d.
- Do this for all elements:
- P: a=c
- Cl: 5a=d
- H: 2b=3c+d
- 4 Solve the system of equations to find the numerical values of the coefficients. The system has several solutions, since there are more variables than equations. It is necessary to find such a solution so that all coefficients have the form of the smallest possible integers.
- To quickly solve a system of equations, assign a numerical value to one of the variables. Suppose a = 1. Let's solve the system and find the values of the remaining variables:
- For P a = c, so c = 1
- For Cl 5a = d, therefore d = 5
- Since for H 2b = 3c + d, we find the value b:
- 2b = 3 (1) + 5
- 2b = 3 + 5
- 2b = 8
- b = 4
- Thus, we have the following coefficients:
- a = 1
- b = 4
- c = 1
- d = 5
Tips
- If you are having difficulty, an online calculator can be used to balance chemical equations. Please note, however, that such a calculator is not allowed to be used during the exam, so do not rely solely on it.
- Remember, sometimes the equation can be simplified! If all coefficients are even divisible by an integer, simplify the equation.
Warnings
- To get rid of fractional coefficients, multiply the entire equation (its left and right sides) by the denominator of the fraction.
- Never use fractions as the coefficients of the chemical equation - there are no half molecules or atoms in chemical reactions.
- In the balancing process, you can use fractions for convenience, but the equation is not balanced as long as there are fractional coefficients in it.