Author:
Lewis Jackson
Date Of Creation:
8 May 2021
Update Date:
25 June 2024

Content
The net ionic equation is an important part of chemistry because it represents only the particles that change in a chemical reaction. They are most commonly used in oxidation-reduction reactions, metabolic reactions, and neutralization reactions of acids - bases. There are three basic steps to writing a net ionic equation: balance the molecular equation, convert it to the full ionic equation (according to how each substance exists in solution), and finally write the net ionic equation.
Steps
Part 1 of 2: Understanding the components of the ionic equation
Know the difference between molecules and ionic compounds. The first step in writing a net ionic equation is to determine the ionic compounds in the reaction. Ionic compounds are those that dissociate ions in an aqueous solution and have an electrical charge. Molecular compounds are compounds that never have an electrical charge. They are formed between two nonmetals and are sometimes referred to as covalent compounds.
- Ionic compounds can be formed between metals and nonmetals, metals and polyatomic ions, or multiple polyatomic ions.
- If you are unsure what the compound is, you can look for the elements in that compound on the periodic table.

Recognize the solubility of a compound. Not all ionic compounds are soluble in aqueous solution, and therefore cannot dissociate into ions. You must identify the solubility of each compound before proceeding with the rest of the equation. Below is a summary of the rules for solubility. Find a solubility spreadsheet for more detailed information and exceptions to these rules.- Follow these rules in the order outlined below:
- All Na, K, and NH salts4 all melt.
- All NO salts3, C2H3O2, ClO3, and ClO4 all melt.
- All Ag, Pb, and Hg salts2 all melt.
- All Cl, Br, and I salts are soluble.
- All CO salts3, O, S, OH, PO4, CrO4, Cr2O7, and SO3 all dissolve (except in a few cases).
- All SO salts4 are dissolved (except in a few cases).
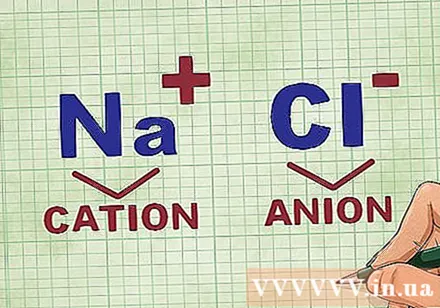
Determination of cations and anions in a compound. Cations are the positive ions in a compound and are usually the metal. The anion is the negative ion in the compound and is non-metallic. Some nonmetals can form cations, but metals always form cations.- For example, in NaCl, Na is the positively charged cation because it is a metal, and Cl is the negatively charged anion because it is nonmetal.

Recognize polyatomic ions in the reaction. Polyatomic ions are charged molecules that are tightly bound together and do not dissociate during chemical reactions. It is important to recognize polyatomic ions because they have a specific charge and do not dissociate. Polyatomic ions can have a positive or negative charge.- If you are studying general chemistry, it is often required to remember some common polyatomic ions.
- Some common polyatomic ions are CO3, NO3, NO2, SO4, SO3, ClO4 and ClO3.
- You can also find many other ions in chemistry books or on the internet.
Part 2 of 2: Writing a net ionic equation
Balance molecular equations. Before writing a net ionic equation, you must make sure your molecular equation is balanced. To balance the equation, you add coefficients in front of the compound so that the atomic number of each element is equal on both sides of the equation.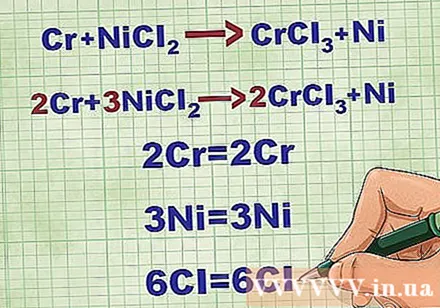
- Write down the number of atoms that make up each compound on both sides of the equation.
- Add a coefficient in front of elements other than oxygen and hydrogen to balance each side.
- Hydrogen atom balance.
- Oxygen atomic balance.
- Recount the number of atoms on each side of the equation to make sure they are balanced.
- For example, Cr + NiCl2 -> CrCl3 + Ni Balanced to 2Cr + 3NiCl2 -> 2CrCl3 + 3Ni.
Identify the state of the compound in the equation. Many times in the problem there are keywords to let you know the state of each compound. There are several rules to help determine the state of an element or compound.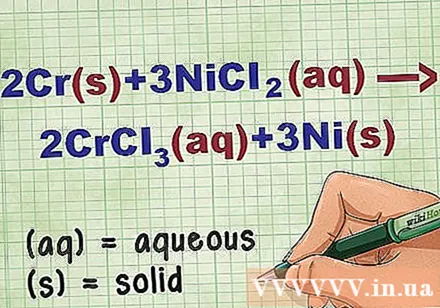
- If no state is provided for the element, use the state found on the periodic table.
- If the compound is called a solution, you can write it as aqueous or (dd).
- If there is water in the equation, you must determine if the ionic compound is water soluble using the solubility spreadsheet. If the solubility is high, the compound will be aqueous (dd), if the solubility is low, the compound will be solid (r).
- Without water, the ionic compound is solid (r).
- If the problem is acidic or basic, then the compound is aqueous (dd).
- For example, 2Cr + 3NiCl2 -> 2CrCl3 + 3Ni. The elemental forms of Cr and Ni are in the solid state. NiCl2 and CrCl3 ionic compounds are soluble, so they are aqueous. Rewrite the equation as: 2Cr(r) + 3NiCl2(dd) -> 2CrCl3(dd) + 3Ni(r).
Determine which compounds will dissociate (dissociate into cations and anions) in solution. When a substance or compound dissociates, it dissociates into a positively charged ion (cation) and a negatively charged ion (anion). These are the components that will be equilibrated at the end of the net ionic equation.
- Solids, liquids, gases, molecular compounds, low solubility ionic compounds, polyatomic ions and weak acids will not dissociate.
- Ionic compounds have a high solubility (use the solubility table) and strong acids will ionize 100% (HCl(dd), HBr(dd), HI(dd), H2SO4(dd), HClO4(dd), and HNO3(dd)).
- Note, although polyatomic ions do not dissociate any more, if they are constituents of that compound, they dissociate from the compound.
Calculate the charge of each ion dissociated from the compound. Remember that metals will form positive ions and nonmetals will form negative ions. Use the periodic table to determine an element's charge. You must also balance the charge of each ion in the compound.
- In this example, NiCl2 dissociated into Ni and Cl while CrCl3 split into Cr and Cl.
- Ni has a charge of 2+ because Cl has a negative charge of one but has 2 atoms. Therefore, we have to balance the 2 negative Cl ions. Cr has a 3+ charge, so we have to balance the 3 negative Cl ions.
- Remember that polyatomic ions have a specific charge.
Write down the complete ionic equation. Anything that dissociates or ionizes (strong acids) separates into two separate ions. The state of the substance remains (dd), but make sure the equation remains balanced.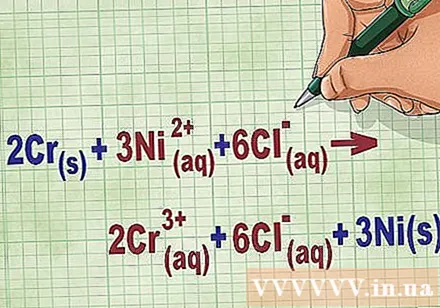
- Solids, liquids, gases, weak acids, and low solubility ionic compounds do not change state or separate into ions. We keep them intact.
- Molecular substances will disperse in solution so their state changes to (dd). Three exceptions are not become (dd) is: CH4(k), C3H8(k), and C8H18(l).
- Continuing with the above example, the full ionic equation looks like this: 2Cr(r) + 3Ni(dd) + 6Cl(dd) -> 2Cr(dd) + 6Cl(dd) + 3Ni(r). When Cl is not in a compound, it is not a bipolar, so we multiply the factor by the number of atoms in the compound to get 6 Cl ions on both sides of the equation.
Remove the equilibrium ions by canceling out the same ions on each side of the equation. You can cancel only if they are exactly the same on both sides (charge, atomic number, etc.). Rewrite the equation without the destructible substances.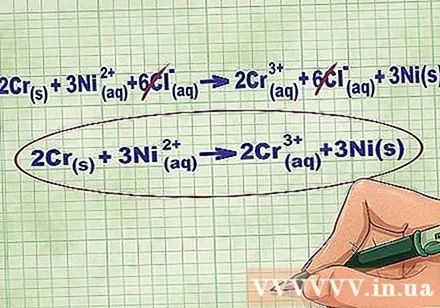
- After completing the example, we have 6 cancelable Cl equilibrium ions on each side. The net ionic equation is 2Cr(r) + 3Ni(dd) -> 2Cr(dd) + 3Ni(r).
- If you are correct then the total charge on the reactant side should be equal to the total charge on the product side in the net ionic equation.
Advice
- Write the states of all substances in the equation, if not you will lose points.