Author:
Peter Berry
Date Of Creation:
20 February 2021
Update Date:
3 July 2024
Content
For a substance in the process of decomposition, the time it takes for the quantity to be halved is called the half-life or half-life. Originally, the term was used to describe the decomposition of a radioactive substance such as uranium or plutonium, however, we can use this term for all substances with a functional decomposition rate. exponential or cyclical. The half-life of all substances can be calculated by the rate of decomposition, a value based on the amount of the original substance and the amount of substance remaining after a specified time period.
Steps
Method 1 of 2: Understanding half-life
About exponential decomposition. The exponential decay follows the formula in it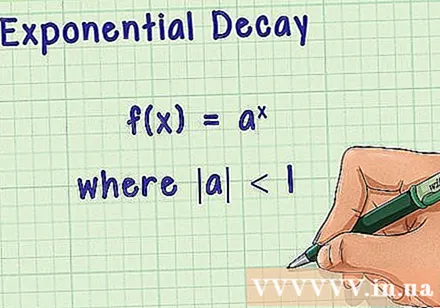
- In other words, as they increase, decrease and gradually approach zero. This is the correlation used to describe the half-life. Considering the half-life, we need, therefore
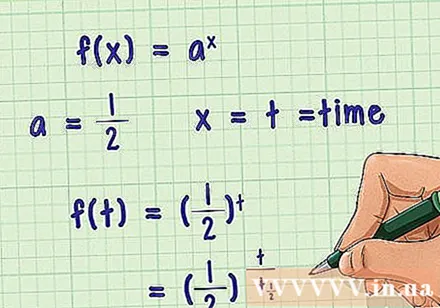
Rewrite the formula as a half cycle. This half-life equation is not dependent on variable but on time- I will be
- At this point, what we need to do is not simply place the values into the variable, but consider the real half-life, in this case, a constant.
- It is then necessary to incorporate half-life into the exponential equation, however, care should be taken when performing this step. In physics, an exponential equation is an isotropic (regardless of direction). We know that the quantity of substances depends on time, so we need to divide the quantity of matter by the half-life - a constant in time units - to get an isotropic quantity.
- Thus, we see that and also have the same units. Therefore, we get the equation outlined below.
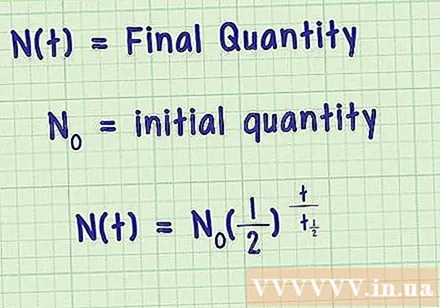
Take into account the initial quality. The equation we are considering is a correlation equation used to determine the percentage of the amount of quality remaining after a period of time compared to the initial quantity of quality. Just add the initial quantity of substance to the equation above and we will get the formula for the half-life of a substance.
Find the half-life. Usually, the above expression includes all the variables that we need to define the half-life. However, if the substance in question is an unknown radioactive material, it is possible to determine its mass before and after a period of time, but its half-life cannot be determined. Therefore, we can expand the half-life according to measurable variables. This is just one way to transform an expression to help you easily identify what you're looking for. Each step of the transformation is as follows: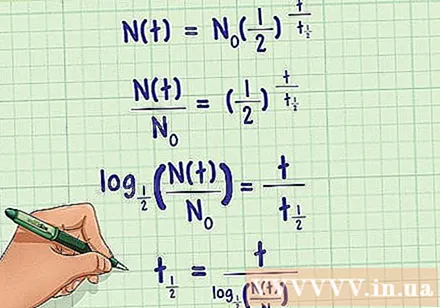
- Divide both sides of the expression by the initial quality
- Taking the base logarithm on both sides of the expression, we get a simpler expression that does not contain an exponent.
- Multiply both sides of the expression by, and then divide both sides by the left side, you get the half-life formula. The result will be in logarithmic form, which you can convert to regular numeric values using a calculator.
Method 2 of 2: Example
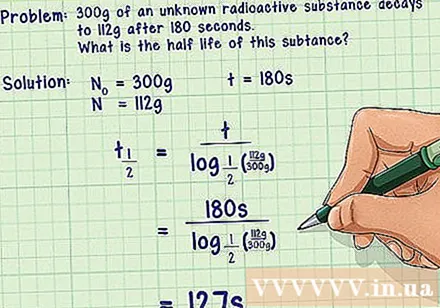
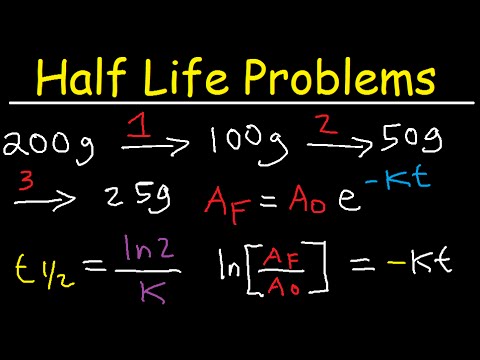
Example 1. Within 180 seconds, an unknown radioactive material decays from its original mass of 300 g to 112 g. What is the half-life of this substance?- The answer: We have the amount of the initial substance is the amount of the remaining substance is the decomposition time.
- The formula for calculating the half-life after the transformation is. You just need to plug the values into the right side of the expression and do the math to get the half-life of the radioactive material in question.
- Check to see if the results are reasonable or not. We find that 112 g is less than half of 300 g, so the substance is at least half decomposed. Since 127 seconds <180 seconds, which means that the substance has passed a half-life, the results we have here are reasonable.
Example 2. A nuclear reactor produces 20 kg of uranium-232. If you know that the half-life of uranium-232 is about 70 years, how long will it take for this uranium-232 to drop to 0.1 kg?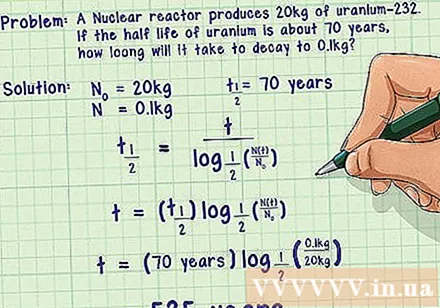
- The answer: We know the quantity of the starting substance is the quantity of the ultimate substance which is the half-life of uranium-232
- Write down the half-life formula based on the half-life.
- Substitute values for variables and compute.
- Remember to always double check to see if the results are reasonable or not.
Advice
- There is another way to calculate half-life using integer bases. In this formula, and will reverse the position in the logarithmic function.
- The half-life is a probabilistic estimate of the amount of time it takes a substance to decay in half rather than an exact calculation. For example, if there is only one atom of substance left, there is no way that the atom will decay to half of the atom after one half-life, but the number of atoms will be zero or remain 1. Quantity the larger the residue, the more accurate the semiconductor period calculation due to the law of probability for extremely large numbers.