Author:
Tamara Smith
Date Of Creation:
19 January 2021
Update Date:
1 July 2024

Content
- To step
- Method 1 of 3: Solution of hydrochloric acid and copper
- Method 2 of 3: Vinegar and bleach
- Method 3 of 3: Vinegar and hydrogen peroxide
- Warnings
- Necessities
- Solution of hydrochloric acid and copper
- Vinegar and bleach
Whether you're conducting a science experiment, incorporating rusty metal into a work of art, or just wanting to try to rust something, rusting metal is very easy if you use the right mixture. This article describes several methods you can choose from.
To step
Method 1 of 3: Solution of hydrochloric acid and copper
 Check whether the metal you are working with will actually rust. Only metals containing iron will rust and some iron alloys will rust slowly or not at all. Stainless steel, an alloy of iron and chrome, is very hard to rust. Cast iron and wrought iron are the easiest to rust.
Check whether the metal you are working with will actually rust. Only metals containing iron will rust and some iron alloys will rust slowly or not at all. Stainless steel, an alloy of iron and chrome, is very hard to rust. Cast iron and wrought iron are the easiest to rust.  Measure out some hydrochloric acid in a plastic bottle. You can easily get hydrochloric acid in low concentrations at hardware stores. Handle it with the utmost care and pour about 60 milliliters into a sturdy plastic bottle. Wear rubber gloves and goggles when doing this.
Measure out some hydrochloric acid in a plastic bottle. You can easily get hydrochloric acid in low concentrations at hardware stores. Handle it with the utmost care and pour about 60 milliliters into a sturdy plastic bottle. Wear rubber gloves and goggles when doing this.  Dissolve a little copper in the hydrochloric acid. When you dissolve copper in hydrochloric acid, you get a rinse that will speed up the rusting process. The best way to dissolve copper in hydrochloric acid is to spiral a short piece of copper wire and leave it immersed in the hydrochloric acid for about a week.
Dissolve a little copper in the hydrochloric acid. When you dissolve copper in hydrochloric acid, you get a rinse that will speed up the rusting process. The best way to dissolve copper in hydrochloric acid is to spiral a short piece of copper wire and leave it immersed in the hydrochloric acid for about a week. - Do not screw the cap on too tightly when you are soaking the copper. The gases released during the chemical reaction will cause pressure to build up in the bottle. Also make sure to put a clear label on the bottle and keep the bottle out of the reach of children or pets.
- You can also use copper coins. Make sure the coin is mostly copper. For example, if you are using US pennies (1 cent coins), know that pennies made after 1982 are only 2.5 percent copper. Pennies made before 1982, however, are 95 percent copper.
 Dilute the copper and hydrochloric acid solution with water. After some of the copper has dissolved in the hydrochloric acid, put on your safety gloves and carefully remove the copper from the mixture. You can discard the copper after removing it from the mixture. Dilute the hydrochloric acid with water at a ratio of about 1 part hydrochloric acid to 50 parts water. If you used 60 milliliters of hydrochloric acid, mix it with about 3.8 liters of water.
Dilute the copper and hydrochloric acid solution with water. After some of the copper has dissolved in the hydrochloric acid, put on your safety gloves and carefully remove the copper from the mixture. You can discard the copper after removing it from the mixture. Dilute the hydrochloric acid with water at a ratio of about 1 part hydrochloric acid to 50 parts water. If you used 60 milliliters of hydrochloric acid, mix it with about 3.8 liters of water.  Thoroughly clean the steel or iron. The hydrochloric acid and copper solution will work best when the metal is very clean. There are specialty products commercially available that are intended to remove scale or corrosion from metal, but cleaning and rinsing with soap and water will generally suffice.
Thoroughly clean the steel or iron. The hydrochloric acid and copper solution will work best when the metal is very clean. There are specialty products commercially available that are intended to remove scale or corrosion from metal, but cleaning and rinsing with soap and water will generally suffice.  Apply the solution of hydrochloric acid and copper to the metal. Apply a thin layer of the solution to the metal and let it air dry. You can apply the hydrochloric acid to the metal with an atomizer or paintbrush, but the hydrochloric acid will quickly attack the metal parts of the atomizer. Wear safety gloves and goggles when applying the hydrochloric acid solution. Work in a well-ventilated area, preferably outdoors.
Apply the solution of hydrochloric acid and copper to the metal. Apply a thin layer of the solution to the metal and let it air dry. You can apply the hydrochloric acid to the metal with an atomizer or paintbrush, but the hydrochloric acid will quickly attack the metal parts of the atomizer. Wear safety gloves and goggles when applying the hydrochloric acid solution. Work in a well-ventilated area, preferably outdoors.  Let the metal rust. Within an hour you should see the metal rusting clearly. You don't have to wipe or rinse the hydrochloric acid solution. It will disappear naturally. If you want a thicker coat of rust, apply another coat of the hydrochloric acid solution.
Let the metal rust. Within an hour you should see the metal rusting clearly. You don't have to wipe or rinse the hydrochloric acid solution. It will disappear naturally. If you want a thicker coat of rust, apply another coat of the hydrochloric acid solution.  Ready.
Ready.
Method 2 of 3: Vinegar and bleach
 Pay attention: do not use chlorine bleach! Mixing chlorine bleach with an acidic agent such as vinegar can produce toxic fumes. First check whether the metal has not been finished with a lacquer or protective layer. This method works best with tin or iron objects. After examining the metal, mix one part vinegar with two parts bleach in a large plastic container or bowl. The amount you use depends on the size of the item you want to rust.
Pay attention: do not use chlorine bleach! Mixing chlorine bleach with an acidic agent such as vinegar can produce toxic fumes. First check whether the metal has not been finished with a lacquer or protective layer. This method works best with tin or iron objects. After examining the metal, mix one part vinegar with two parts bleach in a large plastic container or bowl. The amount you use depends on the size of the item you want to rust.  Place the metal in the container. Unless you only want to rust half of the metal, make sure the metal item is completely submerged. Let the metal sit in the mixture for about thirty minutes. During this time, a nice rust crust will develop on the metal.
Place the metal in the container. Unless you only want to rust half of the metal, make sure the metal item is completely submerged. Let the metal sit in the mixture for about thirty minutes. During this time, a nice rust crust will develop on the metal.  Dry the metal objects with paper towels. You can also dry them with regular towels if you don't mind getting rust stains on them. Also know that if you use paper towels you will get very cool looking paper towels with a rust color after drying. Discard the vinegar and bleach mixture down the drain.
Dry the metal objects with paper towels. You can also dry them with regular towels if you don't mind getting rust stains on them. Also know that if you use paper towels you will get very cool looking paper towels with a rust color after drying. Discard the vinegar and bleach mixture down the drain.  Wait until the items are completely dry before doing anything with them. Make sure items are completely dry before handling them so you don't overexpose your skin to the bleach. When the items are dry, rub away as much rust as you think is necessary. Some people prefer a thick layer of rust, while others like an eroded look.
Wait until the items are completely dry before doing anything with them. Make sure items are completely dry before handling them so you don't overexpose your skin to the bleach. When the items are dry, rub away as much rust as you think is necessary. Some people prefer a thick layer of rust, while others like an eroded look.  Use spray paint to protect the rust on the metal. A matte spray paint usually works well to protect the rust on an object. You can buy spray paint for metal at hardware stores.
Use spray paint to protect the rust on the metal. A matte spray paint usually works well to protect the rust on an object. You can buy spray paint for metal at hardware stores.
Method 3 of 3: Vinegar and hydrogen peroxide
 Protect your workplace if necessary.
Protect your workplace if necessary. Put the metal objects down.
Put the metal objects down.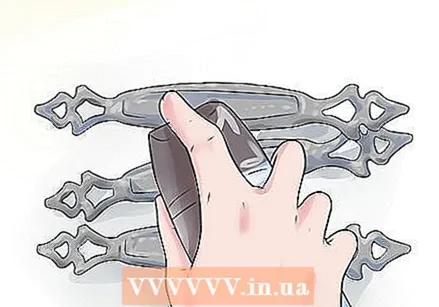 Spray the objects with hydrogen peroxide.
Spray the objects with hydrogen peroxide.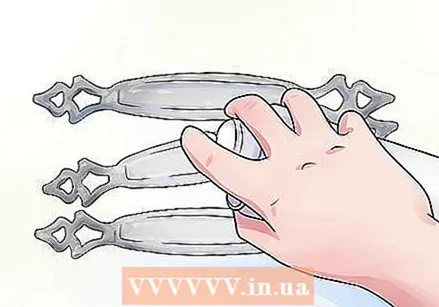 Immediately afterwards spray the items with white vinegar.
Immediately afterwards spray the items with white vinegar.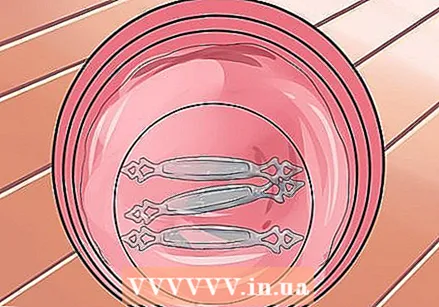 Leave the items for the rest of the day.
Leave the items for the rest of the day.
Warnings
- Always be careful when working with hydrochloric acid, bleach, or hydrogen peroxide. Even in small concentrations, these chemicals can cause skin irritation and mucous membrane irritation.
Necessities
Solution of hydrochloric acid and copper
- Iron or iron alloy
- Rubber gloves
- Safety glasses
- Hydrochloric acid
- Measuring spoon
- Plastic bottle
- Copper wire
- Bucket of 4 liters
- Water
- Soap
- Cloth
- Spray or paint brush
Vinegar and bleach
- Bleach (no chlorine bleach)
- Vinegar
- Plastic container or bowl for mixing
- Pieces of kitchen paper