Author:
Clyde Lopez
Date Of Creation:
21 June 2021
Update Date:
1 July 2024

Content
- Steps
- Method 1 of 2: Calculation of Concentrations Accurately
- Method 2 of 2: Getting Simple Solutions for Practical Purposes
- Warnings
As a result of dilution, the solution becomes less concentrated. Solutions are diluted (diluted) to lower concentrations for a variety of reasons. For example, biochemists dilute concentrated solutions in order to obtain new solutions, which they then use in their experiments. Bartenders, on the other hand, often dilute spirits with softer or juices to get cocktails that taste good. Use the formula to calculate the dilution ratio C1V1 = C2V2where C1 and C2 are the initial and final concentration of the solution, respectively, and V1 and V2 - initial and final volume.
Steps
Method 1 of 2: Calculation of Concentrations Accurately
 1 Determine what you know and what you don't. In chemistry, dilution usually means making a small amount of a solution of known concentration and then diluting it with a neutral liquid (such as water) and thus obtaining a less concentrated solution of a larger volume. This operation is very often used in chemical laboratories, so reagents are stored for convenience in a concentrated form and diluted if necessary. In practice, as a rule, the initial concentration is known, as well as the concentration and volume of the solution to be obtained; wherein unknown volume of concentrated solution to be diluted.
1 Determine what you know and what you don't. In chemistry, dilution usually means making a small amount of a solution of known concentration and then diluting it with a neutral liquid (such as water) and thus obtaining a less concentrated solution of a larger volume. This operation is very often used in chemical laboratories, so reagents are stored for convenience in a concentrated form and diluted if necessary. In practice, as a rule, the initial concentration is known, as well as the concentration and volume of the solution to be obtained; wherein unknown volume of concentrated solution to be diluted. - In another situation, for example, when solving a school problem in chemistry, another quantity can act as an unknown: for example, the initial volume and concentration are given, and it is required to find the final concentration of the final solution with its known volume. In any case, it is helpful to write down the known and unknown quantities before starting the task.
- Let's look at an example. Suppose we need to dilute a solution with a concentration of 5 M to get a solution with a concentration of 1 mM... In this case, we know the concentration of the initial solution, as well as the volume and concentration of the solution to be obtained; not the volume of the initial solution, which must be diluted with water, is known.
- Remember: in chemistry, M is a measure of concentration, also called molarity, which corresponds to the number of moles of the substance per 1 liter of solution.
 2 Plug in known values into formula C1V1 = C2V2. In this formula C1 is the concentration of the initial solution, V1 - its volume, C2 is the concentration of the final solution, and V2 - its volume. From the resulting equation, you can easily determine the desired value.
2 Plug in known values into formula C1V1 = C2V2. In this formula C1 is the concentration of the initial solution, V1 - its volume, C2 is the concentration of the final solution, and V2 - its volume. From the resulting equation, you can easily determine the desired value. - Sometimes it is helpful to put a question mark in front of the quantity you want to find.
- Let's go back to our example. Let's substitute the known values into the equality:
- C1V1 = C2V2
- (5 M) V1 = (1 mm) (1 l). Concentrations have different units of measurement. Let's dwell on this in a little more detail.
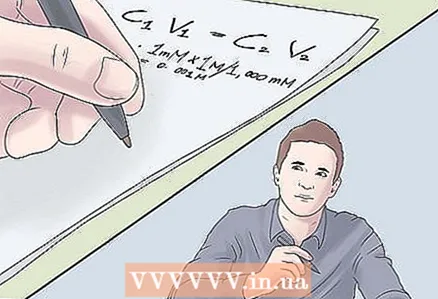 3 Be aware of any difference in units of measurement. Since dilution leads to a decrease in concentration, and often a significant one, sometimes the concentrations are measured in different units. If you miss this, you can be orders of magnitude wrong with the result. Before solving the equation, convert all concentration and volume values to the same units.
3 Be aware of any difference in units of measurement. Since dilution leads to a decrease in concentration, and often a significant one, sometimes the concentrations are measured in different units. If you miss this, you can be orders of magnitude wrong with the result. Before solving the equation, convert all concentration and volume values to the same units. - In our case, two units of concentration are used, M and mM. Let's translate everything into M:
- 1 mM × 1 M / 1.000 mM
- = 0.001 M.
- In our case, two units of concentration are used, M and mM. Let's translate everything into M:
 4 Let's solve the equation. When you have converted all quantities to the same units of measurement, you can solve the equation. To solve it, knowledge of simple algebraic operations is almost always sufficient.
4 Let's solve the equation. When you have converted all quantities to the same units of measurement, you can solve the equation. To solve it, knowledge of simple algebraic operations is almost always sufficient. - For our example: (5 M) V1 = (1 mm) (1 l). Reducing everything to the same units, we solve the equation for V1.
- (5 M) V1 = (0.001 M) (1 L)
- V1 = (0.001 M) (1 L) / (5 M).
- V1 = 0.0002 l, or 0.2 ml.
- For our example: (5 M) V1 = (1 mm) (1 l). Reducing everything to the same units, we solve the equation for V1.
 5 Consider putting your findings into practice. Let's say you have calculated the required value, but you still find it difficult to prepare a real solution. This situation is quite understandable - the language of mathematics and pure science is sometimes far from the real world. If you already know all four quantities in the equation C1V1 = C2V2, proceed as follows:
5 Consider putting your findings into practice. Let's say you have calculated the required value, but you still find it difficult to prepare a real solution. This situation is quite understandable - the language of mathematics and pure science is sometimes far from the real world. If you already know all four quantities in the equation C1V1 = C2V2, proceed as follows: - Measure volume V1 solution concentration C1... Then add the diluting liquid (water, etc.) so that the volume of the solution becomes equal to V2... This new solution will have the required concentration (C2).
- In our example, we first measure 0.2 ml of the stock solution with a concentration of 5 M. Then we dilute it with water to a volume of 1 L: 1 L - 0.0002 L = 0.9998 L, that is, add 999.8 ml of water to it. The resulting solution will have the required concentration of 1 mM.
Method 2 of 2: Getting Simple Solutions for Practical Purposes
 1 Check the information on the packaging. It is often necessary to dilute something in the kitchen or for other household purposes. For example, make orange juice from concentrate.In most cases, the packaging of a reconstituted product contains information on how to do this, often with detailed instructions. When reading the instructions, pay attention to the following:
1 Check the information on the packaging. It is often necessary to dilute something in the kitchen or for other household purposes. For example, make orange juice from concentrate.In most cases, the packaging of a reconstituted product contains information on how to do this, often with detailed instructions. When reading the instructions, pay attention to the following: - the volume of the product used;
- the volume of liquid in which the product must be diluted;
- type of liquid (usually water);
- special breeding instructions.
- Maybe you not you will find information on the exact volume of liquid, since such information is superfluous for an ordinary consumer.
 2 Add the diluting liquid to the concentrated solution. At home, for example in the kitchen, you only need to know the volume of the concentrate used and the approximate final volume. Dilute the concentrate with the required amount of liquid, determined by the volume of the concentrate to be diluted. Wherein:
2 Add the diluting liquid to the concentrated solution. At home, for example in the kitchen, you only need to know the volume of the concentrate used and the approximate final volume. Dilute the concentrate with the required amount of liquid, determined by the volume of the concentrate to be diluted. Wherein: - If, for example, you want to dilute 1 cup of orange juice concentrate to 1/4 of its original concentration, you must add 3 cups water. Thus, the final 4-cup solution will contain one cup of concentrate, or 1/4 of the total.
- A more complicated example: if you want to breed 2/3 cup concentrate to 1/4 of its original concentration, add 2 cups of water, since 2/3 cup is 1/4 of the total liquid of 2 x 2/3 cup.
- Make sure in advance that the prepared containers are sufficient to hold the entire final volume of liquid; use a large cup or bowl.
 3 As a rule, the volume of the concentrate powder can be ignored. Usually the addition of a small amount of powder does not result in any noticeable change in the volume of the liquid. In other words, you can pour the powder into the final volume liquid and stir.
3 As a rule, the volume of the concentrate powder can be ignored. Usually the addition of a small amount of powder does not result in any noticeable change in the volume of the liquid. In other words, you can pour the powder into the final volume liquid and stir.
Warnings
- Adhere to the safety instructions specified by the manufacturer or your company's regulations. This is especially important if you are diluting an acid solution.
- When working with acid solutions, you will need additional dilution and safety instructions.