Content
- Steps
- Method 1 of 3: Finding Atomic Mass Using the Periodic Table of Elements
- Method 2 of 3: Calculating the Atomic Mass of an Individual Atom
- Method 3 of 3: Calculating the relative atomic mass (atomic weight) of an element
- Tips
- What do you need
Atomic mass is the sum of the masses of all protons, neutrons and electrons that make up this or that atom or molecule. Compared to protons and neutrons, the mass of electrons is very small, so it is not taken into account in the calculations. Although this is incorrect from a formal point of view, this term is often used to refer to the average atomic mass of all isotopes of an element. In fact, this is the relative atomic mass, also called atomic weight element. Atomic weight is the average of the atomic masses of all naturally occurring isotopes of an element. Chemists must distinguish between these two types of atomic mass when doing their job - an incorrect atomic mass value can, for example, lead to an incorrect result for the yield of a reaction product.
Steps
Method 1 of 3: Finding Atomic Mass Using the Periodic Table of Elements
 1 Learn how atomic mass is written. Atomic mass, that is, the mass of a given atom or molecule, can be expressed in standard SI units - grams, kilograms, and so on. However, due to the fact that the atomic masses expressed in these units are extremely small, they are often recorded in unified atomic mass units, or abbreviated amu. - atomic mass units. One atomic mass unit is equal to 1/12 of the mass of the standard isotope carbon-12.
1 Learn how atomic mass is written. Atomic mass, that is, the mass of a given atom or molecule, can be expressed in standard SI units - grams, kilograms, and so on. However, due to the fact that the atomic masses expressed in these units are extremely small, they are often recorded in unified atomic mass units, or abbreviated amu. - atomic mass units. One atomic mass unit is equal to 1/12 of the mass of the standard isotope carbon-12. - The atomic mass unit characterizes the mass one mole of a given element in grams... This value is very useful in practical calculations, since it can be used to easily convert the mass of a given number of atoms or molecules of a given substance into moles, and vice versa.
 2 Find the atomic mass in the periodic table. Most standard periodic tables contain the atomic masses (atomic weights) of each element. As a rule, they are shown as a number at the bottom of the cell with the element, under the letters denoting the chemical element. This is usually not an integer, but a decimal fraction.
2 Find the atomic mass in the periodic table. Most standard periodic tables contain the atomic masses (atomic weights) of each element. As a rule, they are shown as a number at the bottom of the cell with the element, under the letters denoting the chemical element. This is usually not an integer, but a decimal fraction. - Note that all the relative atomic masses given in the periodic table for each element are average values. The chemical elements have different isotopes - chemical species that have different masses due to additional or missing neutrons in the atomic nucleus. Therefore, the relative atomic masses listed in the periodic table can be used as an average for the atoms of a particular element, but not as the mass of one atom of a given element.
- The relative atomic masses given in the periodic table are used to calculate the molar masses of atoms and molecules. Atomic masses expressed in amu (as in the periodic table) are essentially dimensionless. However, simply by multiplying the atomic mass by 1 g / mol, we get a useful characteristic of an element - the mass (in grams) of one mole of atoms of this element.
 3 Remember that the periodic table lists the average atomic masses of the elements. As noted earlier, the relative atomic masses indicated for each element in the periodic table are the average of the masses of all isotopes in an atom. This average is valuable for many practical purposes: for example, it is used to calculate the molar mass of molecules made up of several atoms. However, when you are dealing with individual atoms, this value is usually not enough.
3 Remember that the periodic table lists the average atomic masses of the elements. As noted earlier, the relative atomic masses indicated for each element in the periodic table are the average of the masses of all isotopes in an atom. This average is valuable for many practical purposes: for example, it is used to calculate the molar mass of molecules made up of several atoms. However, when you are dealing with individual atoms, this value is usually not enough. - Since the average atomic mass is the average value for several isotopes, the value indicated in the periodic table is not accurate the value of the atomic mass of any single atom.
- The atomic masses of individual atoms must be calculated taking into account the exact number of protons and neutrons in a single atom.
Method 2 of 3: Calculating the Atomic Mass of an Individual Atom
 1 Find the atomic number of a given element or its isotope. The atomic number is the number of protons in the atoms of an element, it never changes. For example, all hydrogen atoms, and only they have one proton. The atomic number of sodium is 11, because its nucleus has eleven protons, while the atomic number of oxygen is eight, since its nucleus has eight protons. You can find the atomic number of any element in the periodic table of Mendeleev - in almost all of its standard versions, this number is indicated above the letter designation of the chemical element. The atomic number is always a positive integer.
1 Find the atomic number of a given element or its isotope. The atomic number is the number of protons in the atoms of an element, it never changes. For example, all hydrogen atoms, and only they have one proton. The atomic number of sodium is 11, because its nucleus has eleven protons, while the atomic number of oxygen is eight, since its nucleus has eight protons. You can find the atomic number of any element in the periodic table of Mendeleev - in almost all of its standard versions, this number is indicated above the letter designation of the chemical element. The atomic number is always a positive integer. - Suppose we are interested in a carbon atom. There are always six protons in carbon atoms, so we know that its atomic number is 6. In addition, we see that in the periodic table, at the top of the cell with carbon (C) is the number "6", indicating that the atomic carbon number is six.
- Note that the atomic number of an element is not uniquely related to its relative atomic mass in the periodic table. Although, especially for the elements at the top of the table, it might appear that the atomic mass of an element is twice its atomic number, it is never calculated by multiplying the atomic number by two.
 2 Find the number of neutrons in the nucleus. The number of neutrons can be different for different atoms of the same element. When two atoms of the same element with the same number of protons have a different number of neutrons, they are different isotopes of that element.Unlike the number of protons, which never changes, the number of neutrons in the atoms of a particular element can often change, so the average atomic mass of an element is written as a decimal fraction with a value lying between two adjacent integers.
2 Find the number of neutrons in the nucleus. The number of neutrons can be different for different atoms of the same element. When two atoms of the same element with the same number of protons have a different number of neutrons, they are different isotopes of that element.Unlike the number of protons, which never changes, the number of neutrons in the atoms of a particular element can often change, so the average atomic mass of an element is written as a decimal fraction with a value lying between two adjacent integers. - The number of neutrons can be determined by the designation of the isotope of the element. For example, carbon-14 is a naturally occurring radioactive isotope of carbon-12. Often the isotope number is indicated as a superscript number in front of the element symbol: C. The number of neutrons is found by subtracting the number of protons from the isotope number: 14 - 6 = 8 neutrons.
- Let's say the carbon atom of interest has six neutrons (C). It is the most abundant isotope of carbon, accounting for about 99% of all atoms of this element. However, about 1% of carbon atoms have 7 neutrons (C). Other types of carbon atoms have more than 7 or less than 6 neutrons and exist in very small quantities.
 3 Add up the number of protons and neutrons. This will be the atomic mass of the given atom. Ignore the number of electrons that surround the nucleus - their total mass is extremely small, so they practically do not affect your calculations.
3 Add up the number of protons and neutrons. This will be the atomic mass of the given atom. Ignore the number of electrons that surround the nucleus - their total mass is extremely small, so they practically do not affect your calculations. - Our carbon atom has 6 protons + 6 neutrons = 12. Thus, the atomic mass of this carbon atom is 12. If this was the isotope "carbon-13", then we would know that it has 6 protons + 7 neutrons = atomic weight 13.
- In fact, the atomic mass of carbon-13 is 13.003355, and this value is more accurate, since it was determined experimentally.
- The atomic mass is very close to the isotope number. For the convenience of calculations, the isotope number is often assumed to be equal to the atomic mass. The experimentally determined values of the atomic mass slightly exceed the isotope number due to the very small contribution from the electrons.
Method 3 of 3: Calculating the relative atomic mass (atomic weight) of an element
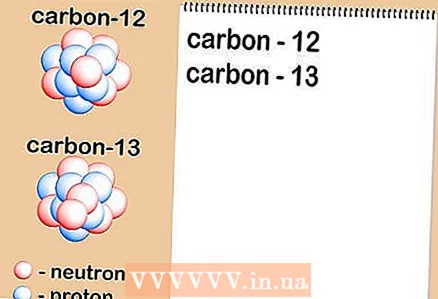 1 Determine which isotopes are in the sample. Chemists often determine the ratio of isotopes in a particular sample using a special instrument called a mass spectrometer. However, during training, these data will be provided to you in the conditions of tasks, control, and so on in the form of values taken from scientific literature.
1 Determine which isotopes are in the sample. Chemists often determine the ratio of isotopes in a particular sample using a special instrument called a mass spectrometer. However, during training, these data will be provided to you in the conditions of tasks, control, and so on in the form of values taken from scientific literature. - In our case, let's say that we are dealing with two isotopes: carbon-12 and carbon-13.
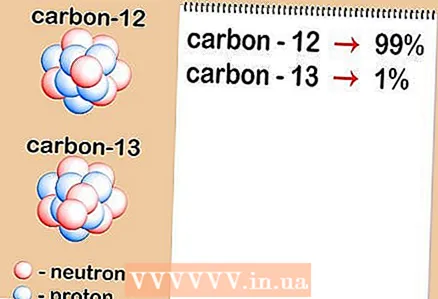 2 Determine the relative content of each isotope in the sample. For each element, different isotopes occur in different proportions. These ratios are almost always expressed as percentages. Some isotopes are very common, while others are very rare - at times so difficult to detect. These quantities can be determined using mass spectrometry or can be found in a handbook.
2 Determine the relative content of each isotope in the sample. For each element, different isotopes occur in different proportions. These ratios are almost always expressed as percentages. Some isotopes are very common, while others are very rare - at times so difficult to detect. These quantities can be determined using mass spectrometry or can be found in a handbook. - Let's say that the concentration of carbon-12 is 99%, and carbon-13 is 1%. Other isotopes of carbon really exist, but in quantities so small that in this case they can be neglected.
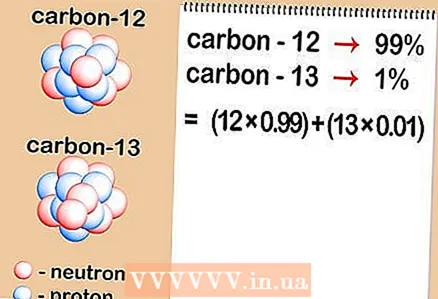 3 Multiply the atomic mass of each isotope by its concentration in the sample. Multiply the atomic mass of each isotope by its percentage (expressed as a decimal fraction). To convert percentages to decimals, simply divide by 100. The resulting concentrations should always add up to 1.
3 Multiply the atomic mass of each isotope by its concentration in the sample. Multiply the atomic mass of each isotope by its percentage (expressed as a decimal fraction). To convert percentages to decimals, simply divide by 100. The resulting concentrations should always add up to 1. - Our sample contains carbon-12 and carbon-13. If carbon-12 is 99% of the sample, and carbon-13 is 1%, then it is necessary to multiply 12 (atomic mass of carbon-12) by 0.99 and 13 (atomic mass of carbon-13) by 0.01.
- The reference books give percentages based on the known amounts of all isotopes of an element. Most chemistry textbooks contain this information in tabular form at the end of the book. For the sample under study, the relative concentrations of isotopes can also be determined using a mass spectrometer.
 4 Add up the results. Sum up the multiplication results you got in the previous step.As a result of this operation, you will find the relative atomic mass of your element - the average value of the atomic masses of the isotopes of the element in question. When considering an element as a whole, rather than a specific isotope of a given element, it is this value that is used.
4 Add up the results. Sum up the multiplication results you got in the previous step.As a result of this operation, you will find the relative atomic mass of your element - the average value of the atomic masses of the isotopes of the element in question. When considering an element as a whole, rather than a specific isotope of a given element, it is this value that is used. - In our example, 12 x 0.99 = 11.88 for carbon-12, and 13 x 0.01 = 0.13 for carbon-13. The relative atomic mass in our case is 11.88 + 0.13 = 12,01.
Tips
- Some isotopes are less stable than others: they decay into atoms of elements with fewer protons and neutrons in the nucleus, releasing particles that make up the atomic nucleus. Such isotopes are called radioactive.
What do you need
- Chemistry Handbook
- Calculator