Author:
Sara Rhodes
Date Of Creation:
12 February 2021
Update Date:
1 July 2024

Content
- Steps
- Part 1 of 2: Determination of the oxidation state according to the laws of chemistry
- Part 2 of 2: Determining the oxidation state without using the laws of chemistry
- Tips
- What do you need
In chemistry, the terms "oxidation" and "reduction" refer to reactions in which an atom or group of atoms loses or, respectively, gains electrons. The oxidation state is a numerical value assigned to one or more atoms that characterizes the number of redistributed electrons and shows how these electrons are distributed between atoms during a reaction. Determination of this value can be both simple and quite complex procedure, depending on the atoms and the molecules consisting of them. Moreover, the atoms of some elements can have several oxidation states. Fortunately, there are simple unambiguous rules for determining the oxidation state, for the confident use of which it is enough to know the basics of chemistry and algebra.
Steps
Part 1 of 2: Determination of the oxidation state according to the laws of chemistry
 1 Determine if the substance in question is elemental. The oxidation state of atoms outside a chemical compound is zero. This rule is true both for substances formed from separate free atoms, and for those that consist of two, or polyatomic molecules of one element.
1 Determine if the substance in question is elemental. The oxidation state of atoms outside a chemical compound is zero. This rule is true both for substances formed from separate free atoms, and for those that consist of two, or polyatomic molecules of one element. - For example, Al(s) and Cl2 have an oxidation state of 0, since both are in a chemically unbound elemental state.
- Note that the allotropic form of sulfur S8, or octacera, despite its atypical structure, is also characterized by a zero oxidation state.
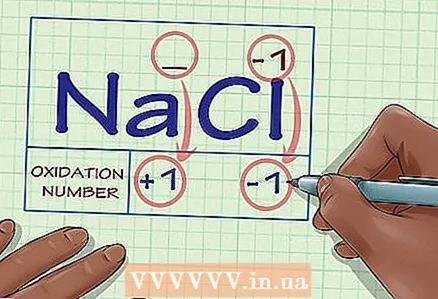 2 Determine if the substance in question is composed of ions. The oxidation state of ions is equal to their charge. This is true both for free ions and for those that are part of chemical compounds.
2 Determine if the substance in question is composed of ions. The oxidation state of ions is equal to their charge. This is true both for free ions and for those that are part of chemical compounds. - For example, the oxidation state of the Cl ion is -1.
- The oxidation state of the Cl ion in the chemical compound NaCl is also -1. Since the Na ion, by definition, has a charge of +1, we conclude that the charge of the Cl ion is -1, and thus its oxidation state is -1.
 3 Please note that metal ions can have several oxidation states. The atoms of many metallic elements can ionize to different amounts. For example, the ion charge of a metal such as iron (Fe) is +2 or +3. The charge of metal ions (and their oxidation state) can be determined by the charges of ions of other elements with which this metal is part of a chemical compound; in the text, this charge is denoted by Roman numerals: for example, iron (III) has an oxidation state of +3.
3 Please note that metal ions can have several oxidation states. The atoms of many metallic elements can ionize to different amounts. For example, the ion charge of a metal such as iron (Fe) is +2 or +3. The charge of metal ions (and their oxidation state) can be determined by the charges of ions of other elements with which this metal is part of a chemical compound; in the text, this charge is denoted by Roman numerals: for example, iron (III) has an oxidation state of +3. - As an example, consider a compound containing an aluminum ion. Total charge of AlCl compound3 is zero.Since we know that Cl ions have a charge of -1, and the compound contains 3 such ions, for the general neutrality of the substance in question, the Al ion must have a charge of +3. Thus, in this case, the oxidation state of aluminum is +3.
 4 The oxidation state of oxygen is -2 (with some exceptions). In almost all cases, oxygen atoms have an oxidation state of -2. There are several exceptions to this rule:
4 The oxidation state of oxygen is -2 (with some exceptions). In almost all cases, oxygen atoms have an oxidation state of -2. There are several exceptions to this rule: - If oxygen is in the elemental state (O2), its oxidation state is 0, as in the case of other elementary substances.
- If oxygen is part of peroxide, its oxidation state is -1. Peroxides are a group of compounds containing a simple oxygen-oxygen bond (i.e. the peroxide anion O2). For example, in the composition of the H2O2 (hydrogen peroxide) oxygen has a charge and oxidation state of -1.
- When combined with fluorine, oxygen has an oxidation state of +2, read the rule for fluorine below.
 5 Hydrogen has an oxidation state of +1, with a few exceptions. As with oxygen, there are also exceptions. As a rule, the oxidation state of hydrogen is +1 (if it is not in the elemental state H2). However, in compounds called hydrides, the oxidation state of hydrogen is -1.
5 Hydrogen has an oxidation state of +1, with a few exceptions. As with oxygen, there are also exceptions. As a rule, the oxidation state of hydrogen is +1 (if it is not in the elemental state H2). However, in compounds called hydrides, the oxidation state of hydrogen is -1. - For example, in H2O The oxidation state of hydrogen is +1 because the oxygen atom has a charge of -2, and two +1 charges are required for overall neutrality. Nevertheless, in the composition of sodium hydride, the oxidation state of hydrogen is already -1, since the Na ion carries a charge of +1, and for the general electroneutrality, the charge of the hydrogen atom (and thus its oxidation state) should be -1.
 6 Fluorine always has an oxidation state of -1. As already noted, the oxidation state of some elements (metal ions, oxygen atoms in peroxides, and so on) can vary depending on a number of factors. The oxidation state of fluorine, however, is invariably -1. This is due to the fact that this element has the greatest electronegativity - in other words, fluorine atoms are the least willing to part with their own electrons and most actively attract foreign electrons. Thus, their charge remains unchanged.
6 Fluorine always has an oxidation state of -1. As already noted, the oxidation state of some elements (metal ions, oxygen atoms in peroxides, and so on) can vary depending on a number of factors. The oxidation state of fluorine, however, is invariably -1. This is due to the fact that this element has the greatest electronegativity - in other words, fluorine atoms are the least willing to part with their own electrons and most actively attract foreign electrons. Thus, their charge remains unchanged.  7 The sum of the oxidation states in a compound is equal to its charge. The oxidation states of all the atoms that make up a chemical compound should add up to the charge of this compound. For example, if a compound is neutral, the sum of the oxidation states of all its atoms should be zero; if the compound is a polyatomic ion with a charge of -1, the sum of the oxidation states is -1, and so on.
7 The sum of the oxidation states in a compound is equal to its charge. The oxidation states of all the atoms that make up a chemical compound should add up to the charge of this compound. For example, if a compound is neutral, the sum of the oxidation states of all its atoms should be zero; if the compound is a polyatomic ion with a charge of -1, the sum of the oxidation states is -1, and so on. - This is a good test method - if the sum of the oxidation states does not equal the total charge of the compound, then you are wrong somewhere.
Part 2 of 2: Determining the oxidation state without using the laws of chemistry
 1 Find atoms that don't have strict rules about their oxidation state. For some elements, there are no firmly established rules for finding the oxidation state. If an atom does not fit any of the rules listed above, and you do not know its charge (for example, the atom is part of a complex, and its charge is not specified), you can determine the oxidation state of such an atom by exclusion. First, determine the charge of all other atoms in the compound, and then, from the known total charge of the compound, calculate the oxidation state of this atom.
1 Find atoms that don't have strict rules about their oxidation state. For some elements, there are no firmly established rules for finding the oxidation state. If an atom does not fit any of the rules listed above, and you do not know its charge (for example, the atom is part of a complex, and its charge is not specified), you can determine the oxidation state of such an atom by exclusion. First, determine the charge of all other atoms in the compound, and then, from the known total charge of the compound, calculate the oxidation state of this atom. - For example, in the compound Na2SO4 the charge of the sulfur atom (S) is unknown - we only know that it is not zero, since sulfur is not in an elementary state. This compound serves as a good example to illustrate the algebraic method for determining the oxidation state.
 2 Find the oxidation states of the rest of the elements that make up the compound. Using the rules described above, determine the oxidation states of the remaining atoms of the compound. Don't forget about the exceptions to the rule for O, H, and so on.
2 Find the oxidation states of the rest of the elements that make up the compound. Using the rules described above, determine the oxidation states of the remaining atoms of the compound. Don't forget about the exceptions to the rule for O, H, and so on. - For Na2SO4, using our rules, we find that the charge (and hence the oxidation state) of the Na ion is +1, and for each of the oxygen atoms it is -2.
 3 Multiply the number of atoms by their oxidation state. Now that we know the oxidation states of all atoms except one, it is necessary to take into account that there may be several atoms of some elements. Multiply the number of atoms of each element (it is indicated in the chemical formula of the compound as a subscript following the symbol of the element) by its oxidation state.
3 Multiply the number of atoms by their oxidation state. Now that we know the oxidation states of all atoms except one, it is necessary to take into account that there may be several atoms of some elements. Multiply the number of atoms of each element (it is indicated in the chemical formula of the compound as a subscript following the symbol of the element) by its oxidation state. - In Na2SO4 we have 2 Na atoms and 4 O atoms. Thus, multiplying 2 × +1, we get the oxidation state of all Na atoms (2), and multiplying 4 × -2 - the oxidation state of the O atoms (-8).
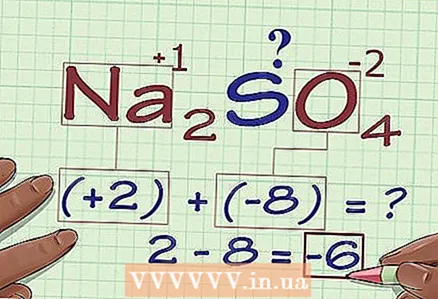 4 Add up the previous results. Summing up the results of multiplication, we get the oxidation state of the compound without taking into account the contribution of the desired atom.
4 Add up the previous results. Summing up the results of multiplication, we get the oxidation state of the compound without taking into account the contribution of the desired atom. - In our example, for Na2SO4 we add 2 and -8 and get -6.
 5 Find the unknown oxidation state from the charge of the compound. You now have all the data to easily calculate the desired oxidation state. Write down an equation, on the left side of which there will be the sum of the number obtained in the previous calculation step and the unknown oxidation state, and on the right side of which there will be the total charge of the compound. In other words, (Sum of known oxidation states) + (desired oxidation state) = (charge of a compound).
5 Find the unknown oxidation state from the charge of the compound. You now have all the data to easily calculate the desired oxidation state. Write down an equation, on the left side of which there will be the sum of the number obtained in the previous calculation step and the unknown oxidation state, and on the right side of which there will be the total charge of the compound. In other words, (Sum of known oxidation states) + (desired oxidation state) = (charge of a compound).- In our case, Na2SO4 the solution looks like this:
- (Sum of known oxidation states) + (desired oxidation state) = (charge of a compound)
- -6 + S = 0
- S = 0 + 6
- S = 6.V Na2SO4 sulfur has an oxidation state 6.
- In our case, Na2SO4 the solution looks like this:
Tips
- In compounds, the sum of all oxidation states must equal the charge. For example, if the compound is a diatomic ion, the sum of the oxidation states of the atoms must equal the total ionic charge.
- It is very useful to be able to use the periodic table and to know where the metallic and non-metallic elements are located in it.
- The oxidation state of atoms in elementary form is always zero. The oxidation state of a single ion is equal to its charge. Elements of group 1A of the periodic table, such as hydrogen, lithium, sodium, in elemental form have an oxidation state of +1; The oxidation state of Group 2A metals, such as magnesium and calcium, is +2 in elemental form. Oxygen and hydrogen, depending on the type of chemical bond, can have 2 different values of the oxidation state.
What do you need
- Periodic table of elements
- Internet access or chemistry reference books
- A sheet of paper, pen or pencil
- Calculator