Author:
Virginia Floyd
Date Of Creation:
7 August 2021
Update Date:
1 July 2024

Content
- Steps
- Method 1 of 2: melt the gold
- Method 2 of 2: Add acid
- Add urea and precipitant =
- Dissolved Gold Acid Test
- Refining gold
- Gold recovery
- Tips
- Warnings
- What do you need
You may want to make money by refining gold at home, or you may be a jeweler who needs to do it. There are a number of methods for refining gold in small quantities, but in any case, precautions must be taken. This article explains how to purify gold using aqua regia.
Steps
Method 1 of 2: melt the gold
 1 Place the gold item in the crucible. Most crucibles are made of graphite that can withstand temperatures high enough to melt the metal.
1 Place the gold item in the crucible. Most crucibles are made of graphite that can withstand temperatures high enough to melt the metal.  2 Place the crucible on a heat-resistant support.
2 Place the crucible on a heat-resistant support. 3 Aim the acetylene torch at the gold object. Maintain the flame until the gold is completely melted.
3 Aim the acetylene torch at the gold object. Maintain the flame until the gold is completely melted.  4 Take the crucible with tongs.
4 Take the crucible with tongs. 5 Divide the gold into small pieces and let them solidify. This is called casting a shot. If you are cleaning a small piece of gold such as a ring, you can do without it.
5 Divide the gold into small pieces and let them solidify. This is called casting a shot. If you are cleaning a small piece of gold such as a ring, you can do without it.
Method 2 of 2: Add acid
 1 Find a suitable container.
1 Find a suitable container.- For every ounce, or roughly 30 grams of gold, you need 300 milliliters of volume.
- Use a thick-walled plastic bucket.
 2 Be sure to use protective equipment.
2 Be sure to use protective equipment.- Wear rubber gloves to protect your hands from acid. Use them in all cases when handling chemicals.
- Use a rubber apron to protect your clothing.
- Protect your eyes with glasses.
- You can use a gauze bandage to protect against toxic fumes.
 3 Place your containers outside in a well-ventilated area. As a result of the reaction of aqua regia with metal, extremely toxic and hazardous fumes will be obtained.
3 Place your containers outside in a well-ventilated area. As a result of the reaction of aqua regia with metal, extremely toxic and hazardous fumes will be obtained.  4 Pour 30 milliliters of nitric acid into your container for each ounce (30 grams) of gold refined. Let the metal react with the acid for 30 minutes.
4 Pour 30 milliliters of nitric acid into your container for each ounce (30 grams) of gold refined. Let the metal react with the acid for 30 minutes.  5 Add 120 milliliters of hydrochloric acid, or hydrochloric acid, for every ounce (30 grams) of gold. Leave the metal in the solution overnight until all acid vapors have dissipated.
5 Add 120 milliliters of hydrochloric acid, or hydrochloric acid, for every ounce (30 grams) of gold. Leave the metal in the solution overnight until all acid vapors have dissipated.  6 Transfer the solution to a different, larger container.
6 Transfer the solution to a different, larger container.- Make sure no particles get into the new container with the acid, as they will contaminate the gold.
- The acid solution should be clear and emerald green in color. If the solution is cloudy, it should be passed through filter paper.
Add urea and precipitant =
- Heat 1 liter of water and add half a kilogram of urea to the water. Continue to heat this solution while bringing it to a boil.

- Add a little urea water solution to the acid.

- When water and urea are added to the acid solution, bubbles will begin to form in it. Add water slowly so that the acid does not spill out of the container.
- The aqueous urea solution will neutralize the nitric acid while the hydrochloric acid remains active.
- Add the selective gold precipitant to a liter of boiling water following the manufacturer's instructions.

- You will have 30 grams of precipitant per 30 grams, or 1 ounce of refined gold.
- Do not lean low over an open container. The vapors of the solution have a very strong and pungent odor.
- Slowly add the resulting aqueous solution of the precipitant to the acid solution.

- The acid will turn a dirty brown color, which indicates the loss of gold particles.
- Wait 30 minutes for all the gold to come out of the solution.
Dissolved Gold Acid Test
- Immerse a stirring stick in the acid solution.

- Use a stick to place a drop of the solution on a paper towel.

- Apply a drop of Precious Metals Detection Liquid to the acid stain. If the stain takes on a purplish hue, you may need to hold the solution for gold precipitation longer.

- Once all the gold is released from the acid solution, pour it into a clean container.

- The acid should be amber in color, and a mud-like sediment forms at the bottom.
- Do not pour out the particles of sediment together with the acid. This dirt is pure gold.
Refining gold
- Pour the sediment remaining in the container with tap water. Stir the water and then let the dirt settle to the bottom.

- Pour the water into the container where you drained the acid earlier.

- Rinse the gold sediment 3-4 more times with water, each time draining the water after it settles to the bottom.

- Wash the gold with aqueous ammonia (ammonia). In this case, you will see how white vapor is released from the sediment. Do not get this vapor in your eyes or inhale it.

- Wash off the remnants of ammonia from the sediment by rinsing it with distilled water.

- Transfer the sediment to a large beaker. Drain off all the distilled water, leaving only a residue.

Gold recovery
- Place the glass on an electric stove. Turn on the stove and gradually heat it up together with the glass so that it does not burst from thermal shock.

- Heat the mud-like precipitate until it looks like a powder.

- Transfer the sediment to a layer of paper towels. Wrap it tightly with these towels and submerge it in rubbing alcohol.

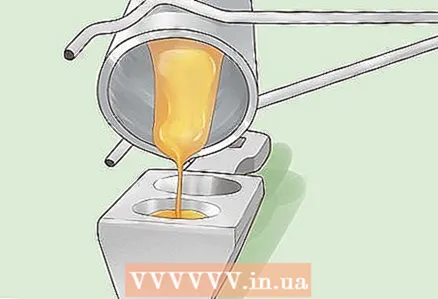
- Place the precipitate in a graphite crucible and melt it. If you did everything correctly, the dirt will turn to 99% pure gold.

- Pour molten gold into a mold and let it cool. You can now take the resulting gold bar to a jeweler or, if you wish, sell it to a jewelery dealer.
Tips
- Refining gold before selling it can help increase revenue significantly.
Warnings
- Become familiar with the laws regarding the purchase, use and disposal of all reagents that you will need to purify gold.
What do you need
- Gold jewelry or other gold items
- Graphite crucible
- Acetylene burner
- At least 3 heavy-walled plastic buckets or large heat-resistant glass chemical containers
- Latex gloves
- Rubber apron
- Protective glasses
- Gauze bandage or respirator
- Nitric acid
- Hydrochloric (hydrochloric) acid
- Urea
- Selective gold precipitant
- Stirring stick
- Paper towels
- Liquid for determination of precious metals
- Ammonia solution (ammonia)
- Distilled water
- Glass beaker
- Electric stove
- Casting mold