Author:
Monica Porter
Date Of Creation:
22 March 2021
Update Date:
1 July 2024

Content
Mass Percent indicates the percentage of each element in a chemical compound. To find the mass percentage, one must know the molar mass of the elements in the compound in grams / mol or the number of grams of the substances that make up the solution. The mass percentage is calculated using a simple formula that divides the mass of the element (or solute) by the mass of the compound (or solution).
Steps
Method 1 of 2: Calculate mass percentage knowing mass
Determine the equation for the percentage of the mass in the mixture. The basic formula for calculating the mass percentage in the mix is: mass percentage = (quality mass / mixed mass) x 100. Finally you have to multiply by 100 to represent the percentage.
- Write an equation when you start solving the problem: mass percentage = (substance mass / mixed mass) x 100.
- The amount of quality will be given in the problem. If the topic is not given, then refer to the following section on how to find mass percentages without knowing weights.
- The mass of the mixture is equal to the total mass of the substances that make up the mixture or solution.

Calculate the mass of the mixture. Once you know the masses of the elements or compounds, all you need to do is add them up to get the mass of the final mixture or solution. This is the denominator in the formula for percentage mass.- Example 1: What is the mass percentage of 5 g of sodium hydroxide when dissolved with 100g of water?
- The mass of the mixture is the total mass of sodium hydroxide and water: 100g + 5g. So the mixed weight is 105g.
- Example 2: Calculate the mass of sodium chloride and water required to make 175g a 15% solution?
- In this example, where you know the mixed mass and the mass percentage, the task asks to find the amount of solute added. The mass of the mixture is 175 g.
- Example 1: What is the mass percentage of 5 g of sodium hydroxide when dissolved with 100g of water?

Determine the mass of the substance to find the percentage mass. When the quiz asks to find the "mass percentage" of a substance, you must find the mass of the substance as a percentage of the total mass of all ingredients. Write down the mass of the substance to find the percentage mass. This is the numerator in the formula for percentage mass.- Example 1: The mass of sodium hydroxide (substance to be found by mass fraction) is 5g.
- Example 2: In this example, the quantity of the substance is looking for the percentage of the unknown mass, and you are looking for it.

Replace the variables with the mass percentages equation. Once you have determined the value of each variable, simply plug them into the equation.- Example 1: mass percentage = (substance mass / mixed mass) x 100 = (5 g / 105 g) x 100.
- Example 2: We need to convert the mass percentage equation to calculate the quantity of unknown quality: quality mass = (percent mass * mixed mass) / 100 = (15 * 175) / 100 .
Calculate the volume percentage. Now that the equation is filled, you only need to calculate the mass percentage. Divide the mass of the substance by the mass of the mixture, then multiply it by 100. This is the mass percentage of the substance in the mixture.
- Example 1: (5/105) x 100 = 0.04761 x 100 = 4.761%. Therefore the mass percentage of 5 g of sodium hydroxide dissolved in 100g of water is 4,761%.
- Example 2: The equation after converting to calculate the quantity of quality is (percentage mass * mixed weight) / 100: (15 * 175) / 100 = (2625) / 100 = 26.25 grams sodium chloride.
- The weight of added water is the mass of the mixture minus the weight of the substance: 175 - 26.25 = 148.75 grams of water.
Method 2 of 2: Calculate mass percentage when mass is unknown
Determine the equation for the percentage of the mass in the compound. The basic formula for calculating the mass percentage in a compound is: mass percentage = (elemental molar mass / compound's molar mass) x 100. Elemental molar mass is the mass of one mole of element while molecular mass is the mass of one mole of compound. Finally, you must multiply by 100 to get the percentage value.
- Write an equation when you start solving the problem: mass percentage = (elemental molar mass / molar mass of compound) x 100.
- The units of the above two values are grams per mol (g / mol).
- When the problem does not give mass, you can use the molar mass to calculate the mass percentage of the element.
- Example 1: Calculate the mass percentage of hydrogen in a water molecule.
- Example 2: Calculate the mass percentage of carbon in a glucose molecule.
Write Chemical formula. If the problem does not cover the chemical formulas for each compound, you will need to write them down. If the problem is for the chemical formula, skip this step and go to "Find the mass of each element" step.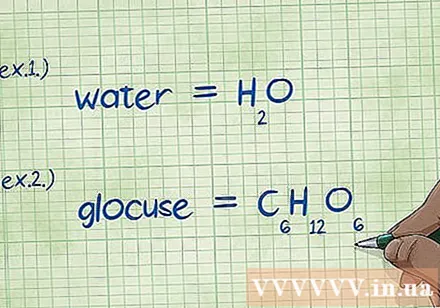
- Example 1: Write the chemical formula for water, H2O.
- Example 2: Write the chemical formula for glucose, C6H12O6.
Find the mass of each element in the compound. Look up the molecular weight of each element in the chemical formula on the periodic table. The elemental mass is usually written below the chemical symbol. Write down the mass of each element in the compound.
- Example 1: We can find that the mass atom of oxygen is 15,9994; and the cubic atom of hydrogen is 1,0079.
- Example 2: We found that the mass atom of carbon is 12,0107; oxygen is 15,9994; and the hydrogen is 1.0079.
Multiply the cubic atom by the molar ratio. Determine the number of moles (molar ratio) of each element in the chemical compound. The molar ratio is calculated as the small number below in the compound's chemical formula. Multiply the cubic atom of each element by the molar ratio.
- Example 1: Hydrogen has a subscript of two while oxygen has a subscript of 1. So multiply the molecular weight of hydrogen by 2, 1,00794 X 2 = 2,01588; and the molecular mass of oxygen is 15,9994 (multiplied by one).
- Example 2: Carbon has a subscript of 6, hydrogen is 12, and oxygen is 6. Multiply the cubic atom of each element by the index below.
- Carbon (12,0107 * 6) = 72,0642
- Hydrogen (1,00794 * 12) = 12,09528
- Oxygen (15,9994 * 6) = 95,9964
Calculate the total mass of the compound. Add the masses of all the elements in the compound. You can calculate the total mass of a compound using the masses expressed in the molar ratio. This number will be the denominator in the percent mass equation.
- Example 1: Add 2,01588 g / mol (the mass of two moles of hydrogen atoms) to 15,9994 g / mol (the mass of one mole of oxygen atoms) gives 18,01528 g / mol.
- Example 2: Add all the weights together: carbon + hydrogen + oxygen = 72,0642 + 12,09528 + 95,9964 = 180,156 g / mol.
Determine the elemental mass to which the mass percentage is to be calculated. When the problem asks for "mass percentage", it means that you must find the mass of a particular element in the compound as a percentage of the total mass of all elements. Determine and write down the mass of the element. This mass is the mass expressed in molar ratio. This number is the numerator of the percent mass equation.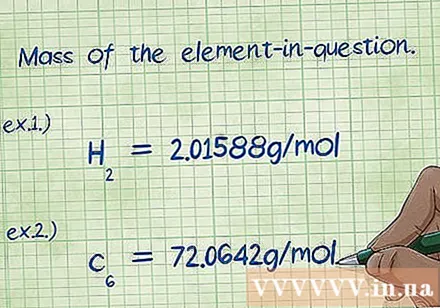
- Example 1: The mass of hydrogen in a compound is 2,01588 g / mol (the mass of two moles of hydrogen atoms).
- Example 2: The mass of carbon in a compound is 72,0642 g / mol (the mass of six moles of carbon atoms).
Replace the variables with the mass percentages equation. After determining the values of each variable, simply replace them with the equation identified in the first step: percentage mass = (elemental molar mass / compound's molar mass) x 100 .
- Example 1: mass percentage = (elemental molar mass / compound's molar mass) x 100 = (2,1588 / 18,1528) x 100.
- Example 2: mass percentage = (elemental molar mass / compound's molar mass) x 100 = (72,0642 / 180,156) x 100.
Calculate the volume percentage. Now that the equation is filled, you only need to calculate the mass percentage. Divide the mass of the element by the total mass of the compound, then multiply it by 100. This is the percentage of the mass of the element in the compound.
- Example 1: mass percent = (2,01588 / 18,01528) x 100 = 0.111189 x 100 = 11.18%. Therefore, the mass percentage of the hydrogen atom in the water molecule is 11.18%.
- Example 2: mass percentage = (elemental molar mass / compound's molar mass) x 100 = (72,0642 / 180,156) x 100 = 0.4000 x 100 = 40.00%. So the mass percentage of the carbon atom in the glucose molecule is 40.00%.