Author:
Christy White
Date Of Creation:
8 May 2021
Update Date:
1 July 2024

Content
- To step
- Method 1 of 3: Part One: Understanding Electron Shells
- Method 2 of 3: Part Two: Finding Valence Electrons in Metals, Except Transition Metals
- Method 3 of 3: Part Three: Finding Valence Electrons in Transition Metals
- Tips
- Necessities
Valence electrons lie in the outer shell of an element. The number of valence electrons in an atom determines the type of chemical bond that this element can form. The best way to find out the number of valence electrons is to use the periodic table of the elements.
To step
Method 1 of 3: Part One: Understanding Electron Shells
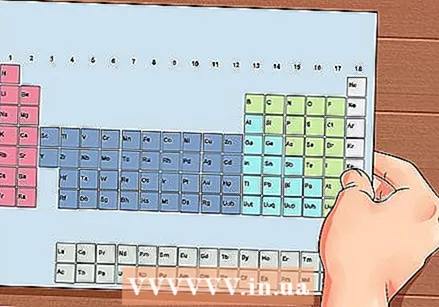 The Periodic Table of the Elements. This is a table with color codes, where in each cell an element is displayed with the atomic number and 1 to 3 letters as a symbol.
The Periodic Table of the Elements. This is a table with color codes, where in each cell an element is displayed with the atomic number and 1 to 3 letters as a symbol. 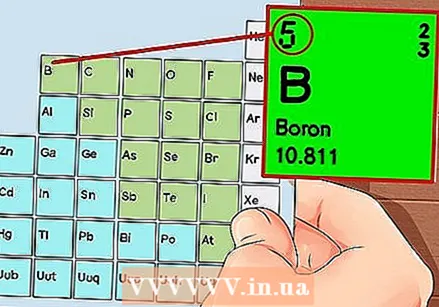 Find the atomic number of the element. The atomic number is above or next to the element's symbol. For example: Boron (B) has an atomic number of 5, which means that it has 5 protons and 5 electrons.
Find the atomic number of the element. The atomic number is above or next to the element's symbol. For example: Boron (B) has an atomic number of 5, which means that it has 5 protons and 5 electrons. 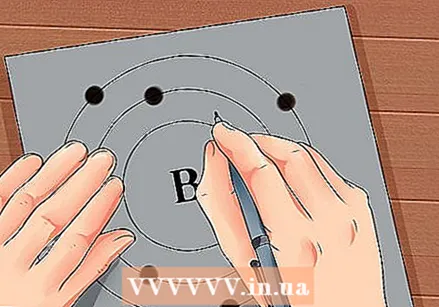 Draw a simple representation of an atom and place the electrons in orbit around the nucleus. These jobs are also called shells or energy levels. The maximum number of electrons that can be in the same shell is fixed, and the shells are filled from the inner to the outer orbit.
Draw a simple representation of an atom and place the electrons in orbit around the nucleus. These jobs are also called shells or energy levels. The maximum number of electrons that can be in the same shell is fixed, and the shells are filled from the inner to the outer orbit. - K Shell (inner): 2 electrons maximum.
- L Shell: 8 electrons maximum.
- M Shell: 18 electrons maximum.
- N Shell: 32 electrons maximum.
- O Shell: 50 electrons maximum.
- P Shell (outer): 72 electrons maximum.
 Find the number of electrons in the outer shell. These are the valence electrons.
Find the number of electrons in the outer shell. These are the valence electrons. - When the valence shell is full, the element is stable.
- If the valence shell is not full then the element is reactive, which means that it can chemically bond with the atom of another element. Each atom shares its valence electrons in an attempt to make the valence shell full.
Method 2 of 3: Part Two: Finding Valence Electrons in Metals, Except Transition Metals
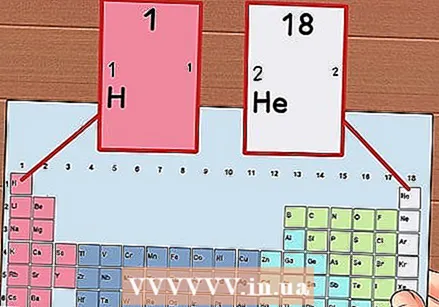 Number each column of the periodic table, from 1 to 18. Hydrogen (H) is at the top of column 1 and Helium (He) at the top of column 18. These are the different groups of elements.
Number each column of the periodic table, from 1 to 18. Hydrogen (H) is at the top of column 1 and Helium (He) at the top of column 18. These are the different groups of elements. 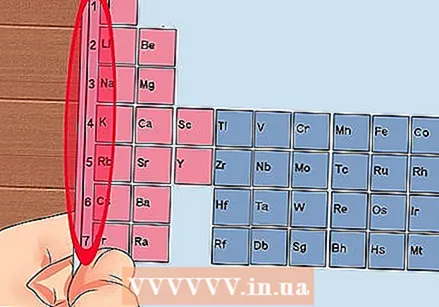 Give each row a number, from 1 to 7. These are the periods of the elements, and they correspond to the number of shells or energy levels of an atom.
Give each row a number, from 1 to 7. These are the periods of the elements, and they correspond to the number of shells or energy levels of an atom. - Hydrogen (H) and Helium (He) both have 1 shell, while Francium (Fr) has 7.
- The lanthanides and actinides are grouped and listed below the main table. All lanthanides belong to Period 6, Group 3 and all actinides belong to Period 7, Group 3.
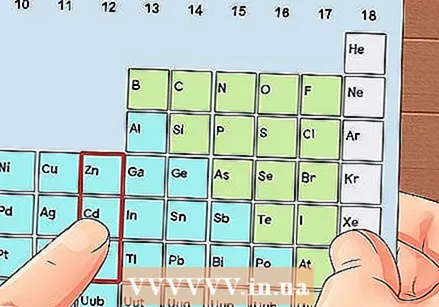 Locate an element that is not a transition metal. Transition metals are in groups 3 through 12. The group numbers of the other metals indicate the number of valence electrons.
Locate an element that is not a transition metal. Transition metals are in groups 3 through 12. The group numbers of the other metals indicate the number of valence electrons. - Group 1: 1 valence electron
- Group 2: 2 valence electrons
- Group 13: 3 valence electrons
- Group 14: 4 valence electrons
- Group 15: 5 valence electrons
- Group 16: 6 valence electrons
- Group 17: 7 valence electrons
- Group 18: 8 valence electrons - except Helium, which has 2
Method 3 of 3: Part Three: Finding Valence Electrons in Transition Metals
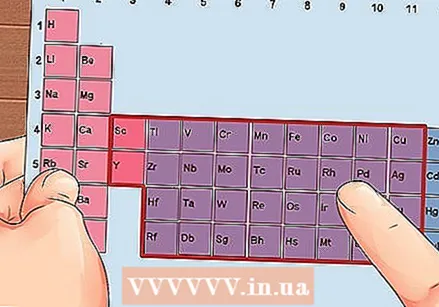 Find an element from groups 3 to 12, the transition metals.
Find an element from groups 3 to 12, the transition metals.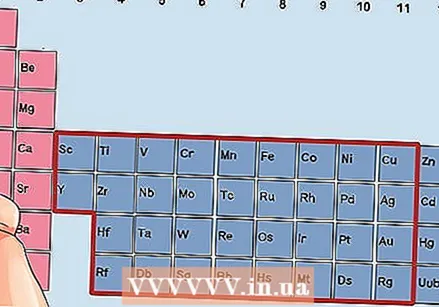 Determine the number of valence electrons based on the group number. These group numbers correspond to a possible number of valence electrons.
Determine the number of valence electrons based on the group number. These group numbers correspond to a possible number of valence electrons. - Group 3: 3 valence electrons
- Group 4: 2 to 4 valence electrons
- Group 5: 2 to 5 valence electrons
- Group 6: 2 to 6 valence electrons
- Group 7: 2 to 7 valence electrons
- Group 8: 2 or 3 valence electrons
- Group 9: 2 or 3 valence electrons
- Group 10: 2 or 3 valence electrons
- Group 11: 1 or 2 valence electrons
- Group 12: 2 valence electrons
Tips
- Transition metals can have valence shells that are not completely full. Determining the exact number of valence electrons in transition metals requires certain principles of quantum theory that are beyond the scope of this paper.
Necessities
- Periodic table of the elements
- Pencil
- Paper