Author:
Roger Morrison
Date Of Creation:
6 September 2021
Update Date:
1 July 2024

Content
- To step
- Method 1 of 2: Make your own sodium acetate
- Method 2 of 2: Try purchased sodium acetate
- Tips
- Warnings
- Necessities
How can ice be hot? If it's not regular ice, of course. Using the same ingredients as lava made from baking soda (baking soda), you can make sodium acetate. By cooling this to below freezing, you get a liquid that will freeze at the slightest activation. During the process of solid crystal formation, heat is released. This is how you make "hot ice cream".
To step
Method 1 of 2: Make your own sodium acetate
 Take a large pan. This should be a clean steel or Pyrex pan, with a capacity of about two liters or more. "Hot ice" is non-toxic, so don't worry about ruining your cooking gear.
Take a large pan. This should be a clean steel or Pyrex pan, with a capacity of about two liters or more. "Hot ice" is non-toxic, so don't worry about ruining your cooking gear. - Do not use a copper pan.
 Add baking soda. Add three tablespoons (45 ml) of baking soda to the pan.
Add baking soda. Add three tablespoons (45 ml) of baking soda to the pan. - You can't use baking soda because it contains other chemicals that interfere with the process.
 Add natural vinegar. Measure out about a liter of natural vinegar and then pour it into the pan little by little. It will foam immediately, so don't pour too quickly or it may overflow.
Add natural vinegar. Measure out about a liter of natural vinegar and then pour it into the pan little by little. It will foam immediately, so don't pour too quickly or it may overflow. - This measurement assumes you are using acetic acid at a concentration of 5%, which is a common concentration for commercial vinegar. However, this does not have to be an exact measurement.
 Wait for the liquid to stop fizzing. The vinegar (acetic acid) and baking soda (sodium bicarbonate) react together to form sodium acetate, along with the carbon dioxide that causes all this fizzing. If it fizzes, stir the liquid well to make sure all of the baking soda is mixed, then wait for the reaction to finish.
Wait for the liquid to stop fizzing. The vinegar (acetic acid) and baking soda (sodium bicarbonate) react together to form sodium acetate, along with the carbon dioxide that causes all this fizzing. If it fizzes, stir the liquid well to make sure all of the baking soda is mixed, then wait for the reaction to finish. 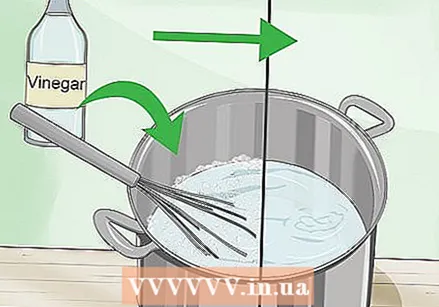 Make sure the liquid is completely clear. If you still see some granules of baking soda, add more vinegar until they are all gone. The baking soda left in the liquid can freeze your "hot ice cream" later in the process, before you want to.
Make sure the liquid is completely clear. If you still see some granules of baking soda, add more vinegar until they are all gone. The baking soda left in the liquid can freeze your "hot ice cream" later in the process, before you want to.  Let it boil until the first trace of a film appears on the surface. Vinegar consists largely of water, which you have to boil away. Once about 90% of the liquid has evaporated - which can take half an hour to boil or more - a crusty film will begin to form on the surface. This means that all excess water is gone and you should turn off the heat as soon as possible. If you let too much of a crust develop, your liquid will become cloudy and not work very well.
Let it boil until the first trace of a film appears on the surface. Vinegar consists largely of water, which you have to boil away. Once about 90% of the liquid has evaporated - which can take half an hour to boil or more - a crusty film will begin to form on the surface. This means that all excess water is gone and you should turn off the heat as soon as possible. If you let too much of a crust develop, your liquid will become cloudy and not work very well. - If it turns very brown and cloudy, add a little more vinegar and cook it again.
- The sodium acetate starts out as "sodium acetate trihydrate," meaning it contains water. When all the water around it is gone, those water molecules begin to evaporate and the sodium acetate becomes "dry sodium acetate".
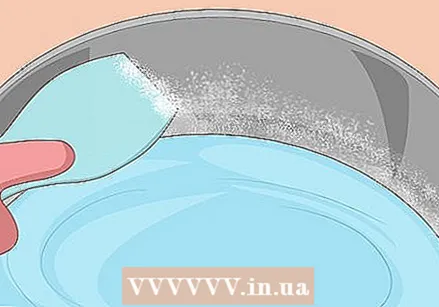 Scrape off the crystals on the side of the pan. As the water level gets lower, you will notice powdery sodium acetate crystals sticking to the inside of the pan. You will need these later, so use a spoon to collect them in a separate container. You can do this anytime while the mixture is cooking.
Scrape off the crystals on the side of the pan. As the water level gets lower, you will notice powdery sodium acetate crystals sticking to the inside of the pan. You will need these later, so use a spoon to collect them in a separate container. You can do this anytime while the mixture is cooking.  Transfer it to a sealable container or bowl. Spoon the liquid into a clean Pyrex bowl or other container that can safely hold warm liquid. Do not allow solid crystals to enter this container. Cover it well.
Transfer it to a sealable container or bowl. Spoon the liquid into a clean Pyrex bowl or other container that can safely hold warm liquid. Do not allow solid crystals to enter this container. Cover it well. - It's a good idea to add 1-2 tablespoons (15-30 ml) of vinegar. The vinegar will help keep the solution in a watery state, rather than making it crust again.
 Cool the container in an ice bath. Wait for the container of sodium acetate to cool to room temperature or below. This should take about 15 minutes in a bowl of ice water, or longer in the refrigerator. The goal is to make the sodium acetate trihydrate "super cool". This means it will drop below its freezing temperature, but still remain liquid.
Cool the container in an ice bath. Wait for the container of sodium acetate to cool to room temperature or below. This should take about 15 minutes in a bowl of ice water, or longer in the refrigerator. The goal is to make the sodium acetate trihydrate "super cool". This means it will drop below its freezing temperature, but still remain liquid. - If the liquid freezes at this stage, it may contain a solid piece of crystal or some other impurity. Add more vinegar, put it back in the pan and try again. This is a difficult process, so it is rare to get it done on your first try.
 Add a little crystallized sodium acetate to your aqueous solution. Use the scrapings from the pan while bringing the solution to a boil. Start with a pinch or two - if those don't work, add more.
Add a little crystallized sodium acetate to your aqueous solution. Use the scrapings from the pan while bringing the solution to a boil. Start with a pinch or two - if those don't work, add more.  Notice how the hot ice forms. The solid sodium acetate provides a seed crystal for any super-cooled acetate to grow from. Since the sodium acetate is already super-cooled and ready to freeze, this should cause a rapid chain reaction, freezing the entire solution. This releases heat, which you can easily feel when you put your hands near the bowl or container.
Notice how the hot ice forms. The solid sodium acetate provides a seed crystal for any super-cooled acetate to grow from. Since the sodium acetate is already super-cooled and ready to freeze, this should cause a rapid chain reaction, freezing the entire solution. This releases heat, which you can easily feel when you put your hands near the bowl or container. - If it doesn't, there is a problem with your solution. Add more vinegar and cook it again - or try the more reliable store-bought method below.
Method 2 of 2: Try purchased sodium acetate
 Look for sodium acetate trihydrate. While this is a cheap, non-toxic substance, it is not widely available in stores. You will probably find it easier to buy it online. You may be able to extract it from a heat pack (which you have to squeeze to activate).
Look for sodium acetate trihydrate. While this is a cheap, non-toxic substance, it is not widely available in stores. You will probably find it easier to buy it online. You may be able to extract it from a heat pack (which you have to squeeze to activate). - Sodium acetate is also sold as "anhydrous sodium acetate" and some suppliers do not specify which form they are referring to. The instructions below cover both forms.
 Place the fabric in boiling water. Place the sodium acetate in a steel pan or Pyrex bowl and then place that container in a pan of boiling water. It should melt into pure liquid sodium acetate trihydrate, or "hot ice".
Place the fabric in boiling water. Place the sodium acetate in a steel pan or Pyrex bowl and then place that container in a pan of boiling water. It should melt into pure liquid sodium acetate trihydrate, or "hot ice". - If the sodium acetate doesn't melt, you bought anhydrous sodium acetate. To convert it to sodium acetate trihydrate, add hot water while it is still in the boiling water bath. For every three grams of sodium acetate, you need about 2 ml of water to completely dissolve the substance.
- Don't use all of your sodium acetate. You need a little for later.
 Cool the fabric immediately. Transfer to a clean container or bowl, cover, and place in an ice bath or refrigerator until it reaches room temperature or below. Make sure that no solid sodium acetate gets into this container or it will freeze too soon.
Cool the fabric immediately. Transfer to a clean container or bowl, cover, and place in an ice bath or refrigerator until it reaches room temperature or below. Make sure that no solid sodium acetate gets into this container or it will freeze too soon.  Touch the cool solution with solid sodium acetate. The solid crystal is a nucleation point, which means that the other sodium acetate molecules can stick around it and expand into a crystal form. In a short time, the entire tub or bowl should look like a block of ice - except it radiates heat!
Touch the cool solution with solid sodium acetate. The solid crystal is a nucleation point, which means that the other sodium acetate molecules can stick around it and expand into a crystal form. In a short time, the entire tub or bowl should look like a block of ice - except it radiates heat! - Other impurities can cause the frostbite if they happen to be the right shape.This means that you can sometimes activate it by touching it with a toothpick or your finger, but solid sodium acetate is the only reliable way.
Tips
- You can make ice sculptures if you pour the solution onto a pinch of the solid crystals. The solution will turn to a solid when it comes into contact with the crystals and will continue to solidify as you pour. The ice will pile up quickly!
- The homemade hot ice cream is more difficult to use and produces less impressive results than the purchased storage method. If you have problems with it, your best bet is to add more vinegar, reduce the water and try again.
- You can melt the solid "hot ice" and repeat the show by re-cooling it. You can easily melt it in the microwave, because you don't have to boil water away anymore.
Warnings
- Hit the solution not until it has cooled down!
Necessities
- Sodium acetate trihydrate (or vinegar and baking soda)
- Medium to large pan (steel or Pyrex)
- Water
- Clean container
- Ice bath (or fridge)