Author:
Frank Hunt
Date Of Creation:
13 March 2021
Update Date:
1 July 2024

Content
- To step
- Method 1 of 3: Using a pH meter
- Method 2 of 3: With litmus paper
- Method 3 of 3: Understanding pH
It is important to measure the pH - the degree of acidity or alkalinity - of water. Water is used by the plants and animals on which we depend, and we ourselves drink it every day. The pH value of water can be an indication of possible contamination, so measuring the pH of water can be an important public health precaution.
To step
Method 1 of 3: Using a pH meter
 Calibrate the probe and meter according to the factory instructions. You may need to calibrate the meter using a substance with a known pH value. The meter can be adjusted according to that substance. If you are going to test water outside of a lab, it is recommended that you perform this calibration a few hours before field testing.
Calibrate the probe and meter according to the factory instructions. You may need to calibrate the meter using a substance with a known pH value. The meter can be adjusted according to that substance. If you are going to test water outside of a lab, it is recommended that you perform this calibration a few hours before field testing. - Rinse the probe with clean water before use. Dry with a clean cloth.
 Take a water sample and pour it into a clean container.
Take a water sample and pour it into a clean container.- The water must be deep enough to submerge the tip of the electrode.
- Leave the sample for a while to allow the temperature to stabilize.
- Measure the temperature of the sample with a thermometer.
 Adjust the meter with the temperature of the sample. The sensitivity of the probe is affected by the water temperature, so the measurement can only be accurate if you enter the temperature data.
Adjust the meter with the temperature of the sample. The sensitivity of the probe is affected by the water temperature, so the measurement can only be accurate if you enter the temperature data. 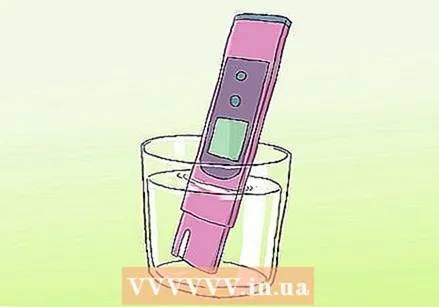 Place the probe in the sample. Wait for the meter to reach equilibrium. The meter is in a steady state when the reading is stable.
Place the probe in the sample. Wait for the meter to reach equilibrium. The meter is in a steady state when the reading is stable.  Read the pH measurement of the sample. The pH meter gives the result on a scale of 0-14. If the water is pure, the value is around 7. Write down your findings.
Read the pH measurement of the sample. The pH meter gives the result on a scale of 0-14. If the water is pure, the value is around 7. Write down your findings.
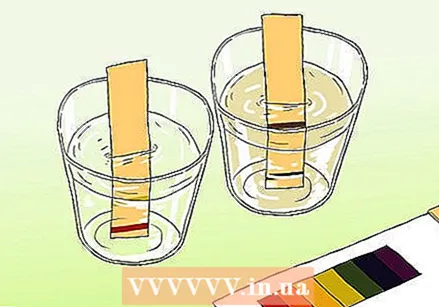
Method 2 of 3: With litmus paper
 Learn the difference between pH paper and litmus paper. You can use pH paper to get an accurate reading of a sample. However, pH paper is not to be confused with regular litmus paper. Both can be used to test for acids and bases, but they differ in important respects.
Learn the difference between pH paper and litmus paper. You can use pH paper to get an accurate reading of a sample. However, pH paper is not to be confused with regular litmus paper. Both can be used to test for acids and bases, but they differ in important respects. - pH strips contain a series of indicator bars that change color when exposed to a solution. The strength of the acids and bases on each bar differs. After the change, the color pattern can be compared with the samples supplied with the kit.
- Litmus paper is a paper strip that contains an acid or base (alkaline). The most common stripes are red (with an acid that reacts with bases) and blue (with a base that reacts with acids). The red stripes turn blue if the substance is alkaline, and the blue stripes turn red if the substance is acidic. Litmus papers can be used as a quick and easy test, but the cheapest varieties do not always give an accurate measure of the strength of the solution.
 Take a sample of the water and pour it into a clean container. The water must be deep enough to submerge the strip.
Take a sample of the water and pour it into a clean container. The water must be deep enough to submerge the strip.  Dip a test strip into the sample. Exposure of a few seconds is enough. The indicator bars on the paper will change color after a few moments.
Dip a test strip into the sample. Exposure of a few seconds is enough. The indicator bars on the paper will change color after a few moments.  Compare the end of the test strip with the color chart that came with the paper. The color or colors on the card must match the color or colors on the test strip. The color map then relates the color patterns to pH levels.
Compare the end of the test strip with the color chart that came with the paper. The color or colors on the card must match the color or colors on the test strip. The color map then relates the color patterns to pH levels.
Method 3 of 3: Understanding pH
 Learn how acids and bases are defined. Acidity and alkalinity (the term used to describe bases) are both defined by the hydrogen ions they donate or take up. An acid is a substance that donates (or "donates") hydrogen ions, and a base is a substance that absorbs extra hydrogen ions.
Learn how acids and bases are defined. Acidity and alkalinity (the term used to describe bases) are both defined by the hydrogen ions they donate or take up. An acid is a substance that donates (or "donates") hydrogen ions, and a base is a substance that absorbs extra hydrogen ions.  Understand the pH scale. The pH number is used to measure the degree of acidity or alkalinity of water-soluble substances. Water normally has the same amount of hydroxide ions (OH−) and hydronium ions (H3O +). The ratio of hydroxide and hydronium ions changes when an acidic or alkaline substance is added to the water.
Understand the pH scale. The pH number is used to measure the degree of acidity or alkalinity of water-soluble substances. Water normally has the same amount of hydroxide ions (OH−) and hydronium ions (H3O +). The ratio of hydroxide and hydronium ions changes when an acidic or alkaline substance is added to the water. - It is usually considered a scale that runs from 0 to 14 (although substances may well fall outside this range). Neutral substances score about 7, acidic substances are under 7 and alkaline substances above 7.
- The pH scale is logarithmic, meaning that integer differences represent a ten-fold difference in acidity or alkalinity. For example, a substance with a pH of 2 is ten times more acidic than a substance with a pH of 3, and 100 times more acidic than a substance with a pH of 4. The scale works the same way with alkaline substances, with any integer being represents a tenfold difference.
 Learn why we test the pH of water. Pure water has a pH of 7, but Dutch tap water usually has a pH of between 7.5 and 8.3. Very acidic water (water with a low pH value) is more likely to dissolve toxic chemicals. These can pollute the water and make it unsafe to drink.
Learn why we test the pH of water. Pure water has a pH of 7, but Dutch tap water usually has a pH of between 7.5 and 8.3. Very acidic water (water with a low pH value) is more likely to dissolve toxic chemicals. These can pollute the water and make it unsafe to drink. - In general, it is advised to test the pH on site. If you take a water sample for lab research, carbon dioxide (CO2) from the air can dissolve in the water. The dissolved carbon dioxide reacts with the ions in the water and increases the acidity in basic or neutral solutions. To avoid carbon dioxide contamination, the water should be tested within two hours of collection.