Author:
Marcus Baldwin
Date Of Creation:
22 June 2021
Update Date:
24 June 2024
Content
Specific heat is the energy required to raise the temperature of one gram of pure material by one degree Celsius. The specific heat capacity of a substance depends on its chemical composition and state of aggregation. The discovery of specific heat has spurred the development of thermodynamics, the science of energy transitions related to heat and system operation. Specific heat and thermodynamics are widely used in chemistry, nuclear engineering and aerodynamics, as well as in everyday life for radiators and car cooling systems. If you would like to know how to calculate the specific heat, follow the instructions below.
Steps
Part 1 of 2: Master the Basics
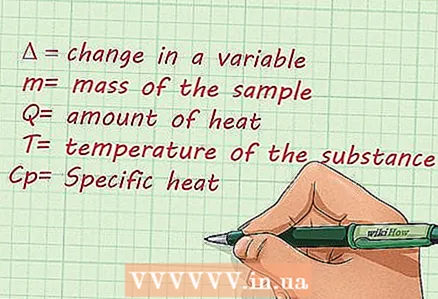 1 Review the values used to calculate the specific heat. It is very important to know the values that are used to calculate the specific heat. You must know what the symbol for each value looks like and understand what it means. The following are the values that are commonly used in an expression to calculate the specific heat of a substance:
1 Review the values used to calculate the specific heat. It is very important to know the values that are used to calculate the specific heat. You must know what the symbol for each value looks like and understand what it means. The following are the values that are commonly used in an expression to calculate the specific heat of a substance: - Delta, or the symbol "Δ", implies a change in value.
- For example, if your first temperature (T1) is 150 ºC and your second (T2) is 20 ºC, then the ΔT, or temperature change, would be 150 ºC - 20 ºC = 130 ºC.
- The mass of the sample is indicated by the letter "m".
- The amount of heat is indicated by the letter "Q". The unit for measuring the amount of heat is "J", or Joule.
- "T" is the temperature of the substance.
- Specific heat is denoted by the letter "Cp».
- Delta, or the symbol "Δ", implies a change in value.
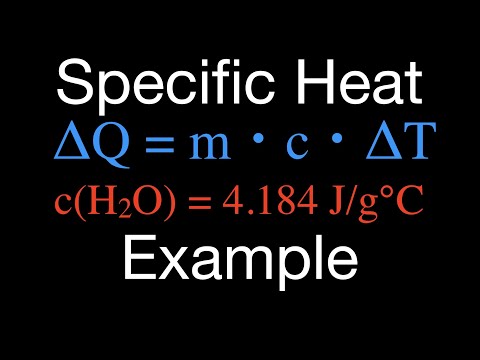
 2 Learn the expression for specific heat. Once you are familiar with the quantities used to calculate the specific heat, you should learn the equation for determining the specific heat of a substance. The formula is: Cp = Q / mΔT.
2 Learn the expression for specific heat. Once you are familiar with the quantities used to calculate the specific heat, you should learn the equation for determining the specific heat of a substance. The formula is: Cp = Q / mΔT. - You can operate with this formula if you want to know the change in the amount of heat instead of the specific heat capacity. This is how it will look:
- ΔQ = mCpΔT
- You can operate with this formula if you want to know the change in the amount of heat instead of the specific heat capacity. This is how it will look:
Part 2 of 2: Calculate Specific Heat
 1 Examine the formula. First, you need to study the expression in order to understand what you need to do to find the specific heat. Let's consider the following task: Determine the specific heat of 350 g of an unknown substance if, when 34,700 J of heat was imparted to it, its temperature rose from 22 to 173 ºC without phase transitions.
1 Examine the formula. First, you need to study the expression in order to understand what you need to do to find the specific heat. Let's consider the following task: Determine the specific heat of 350 g of an unknown substance if, when 34,700 J of heat was imparted to it, its temperature rose from 22 to 173 ºC without phase transitions.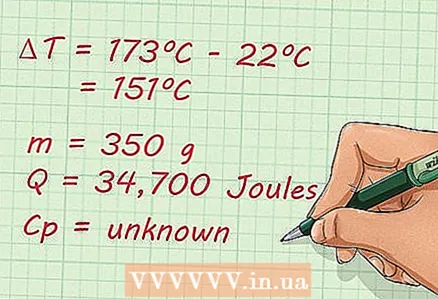 2 Write down known and unknown factors. Once you understand the problem, you can write down all known and unknown variables to better understand what you are dealing with. Here's how to do it:
2 Write down known and unknown factors. Once you understand the problem, you can write down all known and unknown variables to better understand what you are dealing with. Here's how to do it: - m = 350 g
- Q = 34 700 J
- ΔT = 173 ºC - 22 ºC = 151 ºC
- Cp = unknown
 3 Plug the unknown factors into the equation. All values are known except for “Cpc ", therefore it is necessary to substitute all other factors into the original equation and find" Cp". You need to do it like this:
3 Plug the unknown factors into the equation. All values are known except for “Cpc ", therefore it is necessary to substitute all other factors into the original equation and find" Cp". You need to do it like this: - Initial equation: Cp = Q / mΔT
- c = 34,700 J / (350 g x 151 ºC)
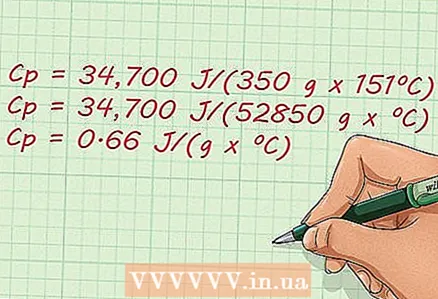 4 Find the answer. Now, after you have substituted the known values in the expression, you just have to perform a few simple arithmetic operations to find out the answer. Specific heat - the final answer - is 0.65657521286 J / (g x ºC).
4 Find the answer. Now, after you have substituted the known values in the expression, you just have to perform a few simple arithmetic operations to find out the answer. Specific heat - the final answer - is 0.65657521286 J / (g x ºC). - Cp = 34.700 J / (350 g x 151 ºC)
- Cp = 34.700 J / (52850 g x ºC)
- Cp = 0.65657521286 J / (g x ºC)
Tips
- Metal heats up faster than water due to its low specific heat.
- When finding the specific heat, reduce the units whenever possible.
- The specific heat of many materials can be found on the Internet to verify your answer.
- Sometimes a calorimeter can be used to study the process of heat transfer during physical or chemical transformations.
- The temperature change, all other things being equal, is more significant for materials with a low specific heat.
- The SI (International System of Units) system unit for specific heat is joule per degree Celsius per gram. In British countries, it is measured in calories per Fahrenheit per pound.
- Learn the formula for calculating the specific heat capacity of food Cp = 4.180 x w + 1.711 x p + 1.928 x f + 1.547 x c + 0.908 x a is the equation for finding the specific heat, where "w" is the percentage of water in the product, "p" is the percentage of proteins, "f" is the percentage of fat, "c" is the percentage of carbohydrates, and "a" is the percentage of inorganic components. The equation takes into account the mass fraction (x) of all solids that make up food. The calculation of the specific heat is given in kJ / (kg x K).