Author:
Clyde Lopez
Date Of Creation:
19 June 2021
Update Date:
1 July 2024

Content
- Steps
- Method 1 of 2: Using TCPO (with different colors)
- Method 2 of 2: Using Luminol
- Tips
- Warnings
- What do you need
- Method One: Using Luminol
- Method Two: Using TCPO
All those videos about the glowing Mountain Dew (Mountain Dew, Russian mountain dew) with peroxide and soda - a complete lie. To make a real glow stick without breaking the finished stick or pouring its contents into a test tube (which is a scam), you need to reveal the chemist in yourself (and spend a few hundred rubles at the same time). If you are still interested, then read on. It will be a lot of fun.
Steps
Method 1 of 2: Using TCPO (with different colors)
 1 Wear protective clothing to protect your eyes and skin. Some chemicals are carcinogenic and, if used improperly, are incredibly dangerous.It is also worth noting that these chemicals are quite expensive and very difficult to obtain. It is best to buy them in bulk, especially if you are going to make a lot of glow sticks. You cannot do without the following things:
1 Wear protective clothing to protect your eyes and skin. Some chemicals are carcinogenic and, if used improperly, are incredibly dangerous.It is also worth noting that these chemicals are quite expensive and very difficult to obtain. It is best to buy them in bulk, especially if you are going to make a lot of glow sticks. You cannot do without the following things: - Latex gloves
- Vented glasses (laboratory glasses)
- Long sleeves
- Respirator
- Clean and tidy work surface
- Clean glass tubes or flasks with lids.
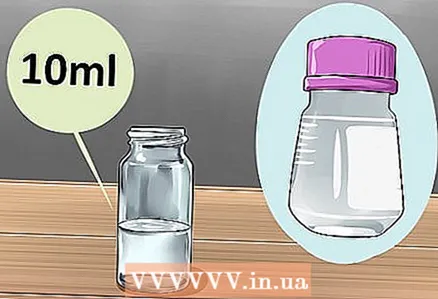 2 Start with 10 ml of diethyl phthalate solution. This is the base that will make up most of the liquid in the glow stick and hold the substances that will glow, while simultaneously enhancing their reaction. Start with 10 ml of diethyl phthalate, but you can double this amount or halve this amount if the stick needs to be made larger or smaller accordingly. Diethyl phthalate is similar to water, but the chemical reaction required to glow will not occur in water.
2 Start with 10 ml of diethyl phthalate solution. This is the base that will make up most of the liquid in the glow stick and hold the substances that will glow, while simultaneously enhancing their reaction. Start with 10 ml of diethyl phthalate, but you can double this amount or halve this amount if the stick needs to be made larger or smaller accordingly. Diethyl phthalate is similar to water, but the chemical reaction required to glow will not occur in water. - To find these chemicals, you have to do a little digging on the internet.
- If you double the amount of solution, then the amount of subsequent components should also be doubled.
 3 To add color to the stick, add 3 mg of the selected phosphor. In this case, you cannot use ordinary or food coloring, so be sure to take a phosphor. The stick will not glow exactly the same color as the phosphor itself, so follow the instructions below to get the color you want. To get the color you want, use a wide selection of phosphors:
3 To add color to the stick, add 3 mg of the selected phosphor. In this case, you cannot use ordinary or food coloring, so be sure to take a phosphor. The stick will not glow exactly the same color as the phosphor itself, so follow the instructions below to get the color you want. To get the color you want, use a wide selection of phosphors: - 9,10-bis- (phenylethynyl) anthracene gives green color
- Rubren gives yellow color
- 9,10-diphenylanthracene gives blue color
- Rhodamine B produces a red color (note: rhodamine degrades quickly, so redness fades quickly)
- Mix half of the yellow phosphor and half of the blue for a white color.
 4 Add 50 mg TCPO to the colored solution. TCPO is bis (2,4,6-trichlorophenyl) oxalate, which will also need to be purchased. This substance is quite expensive, but if you have experience with chemicals, it can be made for a relatively small amount of money. Otherwise, we do not recommend making it by hand.
4 Add 50 mg TCPO to the colored solution. TCPO is bis (2,4,6-trichlorophenyl) oxalate, which will also need to be purchased. This substance is quite expensive, but if you have experience with chemicals, it can be made for a relatively small amount of money. Otherwise, we do not recommend making it by hand. - In this method, TCPO is used instead of luminol. This is the component due to which the solution glows for several hours.
- TCPO is incredibly carcinogenic and should be handled with great care. Don't inhale it.
 5 Add 100 mg sodium acetate to the solution. If you don't have one, then add a 1: 1 mixture of sodium bicarbonate (baking soda) and sodium salicylate. Then close the flask with a lid and shake well.
5 Add 100 mg sodium acetate to the solution. If you don't have one, then add a 1: 1 mixture of sodium bicarbonate (baking soda) and sodium salicylate. Then close the flask with a lid and shake well.  6 Finally, add 3 ml of 30% hydrogen peroxide solution to the composition. Peroxide should be added last, as a chemical reaction will then begin. Close the flask with a lid, shake it well and turn off the light. Now you have a stick / test tube / flask brightly glowing in your hands.
6 Finally, add 3 ml of 30% hydrogen peroxide solution to the composition. Peroxide should be added last, as a chemical reaction will then begin. Close the flask with a lid, shake it well and turn off the light. Now you have a stick / test tube / flask brightly glowing in your hands. - Hydrogen peroxide acts only as a catalyst and is not a necessary part of the reaction, so very little is needed.
- To make the store stick glow, it needs to be broken in half. The crackling sound you hear is the sound of a glass bubble of hydrogen peroxide breaking.
 7 Add more TCPO and sodium acetate to prolong the reaction. If you like, experiment with the number of components to see which will give the best result. The reaction occurs because the combination of TCPO and sodium acetate releases energy, after which they decompose. This energy then comes into contact with the phosphor, which converts the energy into a glow. A larger volume of components will lead to the release of more energy, and therefore a longer reaction.
7 Add more TCPO and sodium acetate to prolong the reaction. If you like, experiment with the number of components to see which will give the best result. The reaction occurs because the combination of TCPO and sodium acetate releases energy, after which they decompose. This energy then comes into contact with the phosphor, which converts the energy into a glow. A larger volume of components will lead to the release of more energy, and therefore a longer reaction.
Method 2 of 2: Using Luminol
 1 Wear safety glasses. In addition, you should also wear gloves to protect your hands. When conducting this experiment, you should not wear formal clothes.Wear old clothes or overalls on top of your belongings to protect them. Some components are quite dangerous, and the experiment itself should not be carried out by children!
1 Wear safety glasses. In addition, you should also wear gloves to protect your hands. When conducting this experiment, you should not wear formal clothes.Wear old clothes or overalls on top of your belongings to protect them. Some components are quite dangerous, and the experiment itself should not be carried out by children! - In this method, we will work with a solution with a pH of about 12. That is, it must not be swallowed, poked into the eyes, bathed in, or allowed to come into contact with the skin. All clear? Let's continue then.
 2 Take a mixing bowl, pour one liter of distilled water into it and add 50 ml to it hydrogen peroxide. It's better to use a ceramic bowl, but a plastic one will work as well. Use a funnel, beaker and syringe to accurately calculate the required amount of components and do not bring them close to the body.
2 Take a mixing bowl, pour one liter of distilled water into it and add 50 ml to it hydrogen peroxide. It's better to use a ceramic bowl, but a plastic one will work as well. Use a funnel, beaker and syringe to accurately calculate the required amount of components and do not bring them close to the body. - Hydrogen peroxide will replace nitrogen atoms with oxygen atoms in luminol. When this happens, a violent chemical reaction will begin and electrons will begin to scatter everywhere. What will all this lead to? That's right, to glow.
 3 Take a second bowl, pour 1 liter of distilled water into it and add 0.2 grams of luminol, 4 grams of sodium carbonate, 0.4 grams of sulfate copper (copper sulfate) and 0.5 grams of ammonium carbonate. Never touch the luminol. To be safe and to make the mixing process easier, use a funnel for this. Unfortunately, these dangerous chemicals will not float as freely in the air as shown in the picture.
3 Take a second bowl, pour 1 liter of distilled water into it and add 0.2 grams of luminol, 4 grams of sodium carbonate, 0.4 grams of sulfate copper (copper sulfate) and 0.5 grams of ammonium carbonate. Never touch the luminol. To be safe and to make the mixing process easier, use a funnel for this. Unfortunately, these dangerous chemicals will not float as freely in the air as shown in the picture. - Unless you are a coroner or one of the crazy spies / criminologists, the chances that all of these chemicals will be lying around in your house are extremely small. If you are looking to start your own glow stick business (there are even worse ideas), visit the Reactor websites for Alfa Aesar products, or visit Sigma Aldrich (in Russian) and search here for the components you need.
- Mix everything well. Do not interfere with your hands, use a metal or plastic cutlery or something similar.
 4 Clean containers and dry thoroughly. It is very important that the tubes for your chopsticks are clean. The last thing you need is for different substances to interfere with the reaction, because of which the solution will glow.
4 Clean containers and dry thoroughly. It is very important that the tubes for your chopsticks are clean. The last thing you need is for different substances to interfere with the reaction, because of which the solution will glow.  5 Place the correct lid next to each container. This will allow you to quickly close the container as soon as you fill it. The liquid, of course, will not grow legs, and it will not run away from you, but still.
5 Place the correct lid next to each container. This will allow you to quickly close the container as soon as you fill it. The liquid, of course, will not grow legs, and it will not run away from you, but still. 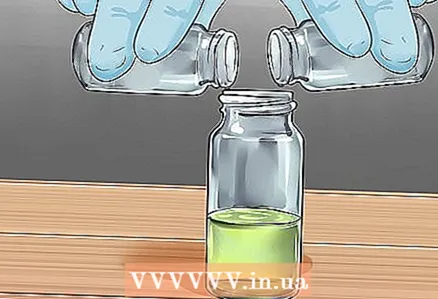 6 Combine equal parts of the first and second solution in containers, then close them. Close the containers tightly with the lids, then shake them well. Then turn off the lights!
6 Combine equal parts of the first and second solution in containers, then close them. Close the containers tightly with the lids, then shake them well. Then turn off the lights! - If the liquid does not start to glow, then something has gone wrong. Do it all over again!
 7 Observe how the chemicals create a colored glow. Take your glow sticks to the party and sell them to your friends for big bucks! But do it quickly, as they won't glow for long. Have all your expectations collapsed? Then use a different method!
7 Observe how the chemicals create a colored glow. Take your glow sticks to the party and sell them to your friends for big bucks! But do it quickly, as they won't glow for long. Have all your expectations collapsed? Then use a different method! - The reaction, which is formed from the combination of luminol and hydrogen peroxide, does not last long, about a few minutes. If you want to make a stick that will glow for several hours, move on to the next method (of course, it will be much easier if you have access to a laboratory, but it is still worth mentioning).
Tips
- Luminol is the compound that makes our blood glow. You can find it in chemical stores, on the Internet, or in a kid's spy kit.
- Sodium carbonate, ammonium carbonate and copper sulfate pentahydrate are all white powders. You can buy them at chemical stores.
- Of course, it is much easier and cheaper to buy the glow sticks yourself, unless you buy all the components in bulk.
- This process is pretty messy. Spread out newspapers or carry it out in a room that can then be easily cleaned up.
Warnings
- Supervise children when they play with glow sticks. They may want to break it apart or swallow the contents, which could seriously harm them.
- When working with chemicals always wear safety glasses.
- These are pretty serious techniques for making glow sticks. This article does not cover simpler methods simply because they do not exist. If you just want to fool around, then you better leave the process of creating glow sticks to professionals. These substances are very dangerous.
- Wear gloves. Do not touch or swallow luminol.
- Copper sulfate is very toxic, so be careful.
What do you need
Method One: Using Luminol
- 2 large ceramic mixing bowls
- Clean containers such as test tubes or glass flasks with lids
- 2 liters of distilled water
- 50 ml of 30% hydrogen peroxide solution
- 0.2 grams of luminol (do not touch it; put on gloves and pour it from the container)
- 4 grams of sodium carbonate
- 0.5 grams of ammonium carbonate
- 0.4 grams of copper sulfate pentahydrate
- Protective glasses
- Gloves
- Funnel and other measuring devices
Method Two: Using TCPO
- 10 ml diethyl phthalate
- 3 mg phosphor
- 50 mg TCPO (bis [2,4,6-trichlorophenyl] oxalate)
- 100 mg sodium acetate
- 3 ml of 30% hydrogen peroxide solution
- Clean containers with lids
- Protective glasses
- Gloves
- Funnel and other measuring devices