Author:
Helen Garcia
Date Of Creation:
17 April 2021
Update Date:
1 July 2024
Content
- Steps
- Method 1 of 2: Calculating Normality via Molarity
- Method 2 of 2: Calculating Normality in Equivalent Mass
- Tips
- What do you need
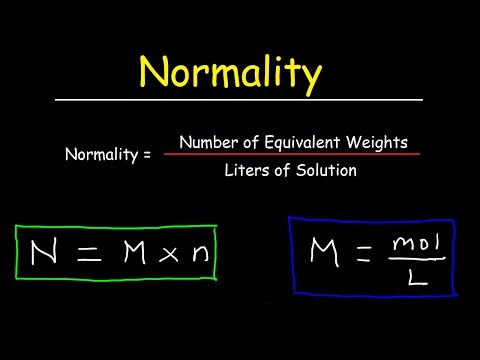
Normality indicates the concentration of acid or alkali in a solution. To find out the normality of a solution, both molarity and the equivalent mass of the molecule can be used in the calculations. If you choose to use molarity, use the formula N = M (n), where M is molarity and n is the number of hydrogen or hydroxide molecules. If you decide to use the equivalent mass, use the formula N = eq ÷ V, where eq is the number of equivalents and V is the volume of the solution.
Steps
Method 1 of 2: Calculating Normality via Molarity
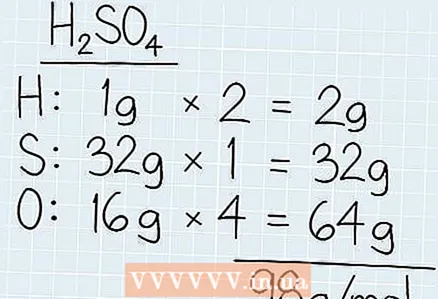 1 Add up the molar mass of all components of the solution. Find the elements of the chemical formula on the periodic table to find out their atomic mass, which corresponds to the molar mass. Write down the molar mass of each element and multiply it by the number of those elements. Add up the molar mass of all components to find the total molar mass.
1 Add up the molar mass of all components of the solution. Find the elements of the chemical formula on the periodic table to find out their atomic mass, which corresponds to the molar mass. Write down the molar mass of each element and multiply it by the number of those elements. Add up the molar mass of all components to find the total molar mass. - For example, if you need to know the molar mass of sulfuric acid (H2SO4), find out the molar mass of hydrogen (1 g), sulfur (3 g) and oxygen (16 g).
- Multiply the mass by the number of components in the composition. In our example, there are 2 hydrogen atoms and 4 oxygen atoms. The total molar mass of hydrogen is 2 x 1 g = 2 g. The molar mass of oxygen in this solution will be 4 x 16 g = 64 g.
- Add all molar masses together. You get 2 g + 32 g + 64 g = 98 g / mol.
- If you already know the molarity of the solution you are looking for, go directly to Step 4.
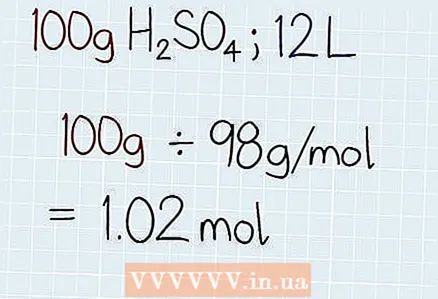 2 Divide the actual weight of the solution by the molar weight. Find out the actual weight of the solution. It will be indicated either on the container with the solution, or in the task itself.Then divide the mass of the solution by the total molar mass found earlier. The result will be the number of moles in the solution, after which “mole” should be written.
2 Divide the actual weight of the solution by the molar weight. Find out the actual weight of the solution. It will be indicated either on the container with the solution, or in the task itself.Then divide the mass of the solution by the total molar mass found earlier. The result will be the number of moles in the solution, after which “mole” should be written. - For example, if you are trying to find out the normality of 100 g H2SO4dissolved in 12 liters of liquid, use the actual mass and divide by molar. As a result, you will get: 100 g ÷ 98 g / mol = 1.02 mol.
- 1 mole is equal to 6.02 x 10 atoms or molecules of a solution.
 3 Divide the result by the volume of the solution in liters to find out the molarity. Take the number of moles in the solution just calculated and divide it by the total volume of the solution to be measured. As a result, you will know the molarity (M), with which you can find out the concentration of the solution.
3 Divide the result by the volume of the solution in liters to find out the molarity. Take the number of moles in the solution just calculated and divide it by the total volume of the solution to be measured. As a result, you will know the molarity (M), with which you can find out the concentration of the solution. - Based on our example, we get the following formula: 1.02 mol ÷ 12 L = 0.085 M.
Advice: be sure to convert the volume of the solution to liters, if you have not already done so. Otherwise, you will get the wrong answer.
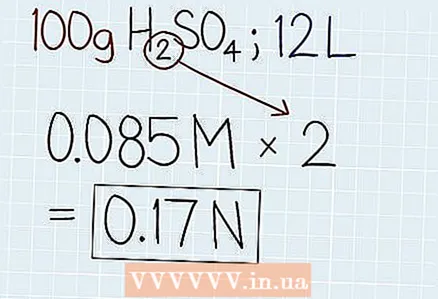 4 Multiply molarity by the number of hydrogen or hydroxide molecules. Take a look at the chemical formula to find out the number of hydrogen atoms (H) in an acid or hydroxide molecules in (OH) in the base. Then multiply the molarity of the solution by the number of hydrogen or hydroxide molecules in that solution to find the normal concentration, or normality. At the end of your answer, write the abbreviation "N".
4 Multiply molarity by the number of hydrogen or hydroxide molecules. Take a look at the chemical formula to find out the number of hydrogen atoms (H) in an acid or hydroxide molecules in (OH) in the base. Then multiply the molarity of the solution by the number of hydrogen or hydroxide molecules in that solution to find the normal concentration, or normality. At the end of your answer, write the abbreviation "N". - In our example, sulfuric acid (H2SO4) 2 hydrogen atoms. So the formula will be like this: 0.085 M x 2 = 0.17 N.
- In another example, sodium hydroxide (NaOH) with a molarity of 2 M has only 1 hydroxide molecule. Therefore, the formula will be as follows: 2 M x 1 = 2 N.
Method 2 of 2: Calculating Normality in Equivalent Mass
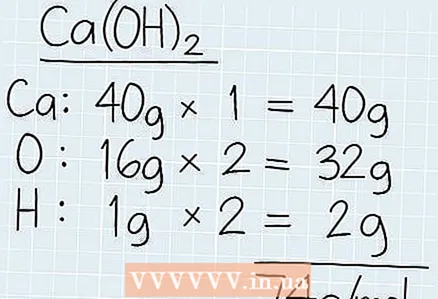 1 Find out the total molar mass of the solution. Take a look at the chemical formula of the solution and find each element on the periodic table. Write down the molar mass of each element and multiply it by the number of those elements in the formula. Add all molar masses together to find the total molar mass in grams.
1 Find out the total molar mass of the solution. Take a look at the chemical formula of the solution and find each element on the periodic table. Write down the molar mass of each element and multiply it by the number of those elements in the formula. Add all molar masses together to find the total molar mass in grams. - For example, if you want to know the molar mass of Ca (OH)2, then find out the molar mass of calcium (40 g), oxygen (16 g) and hydrogen (1 g).
- In the formula there are 2 atoms of oxygen and hydrogen. The total mass of oxygen will be: 2 x 16 g = 32 g. The molar mass of hydrogen will be: 2 x 1 g = 2 g.
- Add all molar masses together to get 40 g + 32 g + 2 g = 74 g / mol.
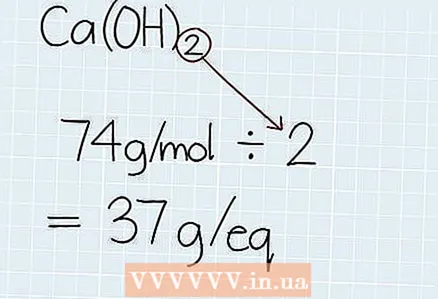 2 Divide the molar mass by the number of hydrogen or hydroxide molecules. Find out the number of hydrogen atoms (H) in an acid or hydroxide (OH) molecules in the base. Divide the total molar mass just obtained by the number of atoms or molecules to find the weight of 1 equivalent, which will be equal to the mass of 1 mole of hydrogen or hydroxide. At the end of your answer, write the abbreviation "G.-e." meaning the mass of the equivalent.
2 Divide the molar mass by the number of hydrogen or hydroxide molecules. Find out the number of hydrogen atoms (H) in an acid or hydroxide (OH) molecules in the base. Divide the total molar mass just obtained by the number of atoms or molecules to find the weight of 1 equivalent, which will be equal to the mass of 1 mole of hydrogen or hydroxide. At the end of your answer, write the abbreviation "G.-e." meaning the mass of the equivalent. - In our example, Ca (OH)2 2 two hydrogen molecules, which means that the mass of the equivalent will be equal to 74 g / mol ÷ 2 = 37 G.-e.
 3 Divide the actual weight of the solution by the equivalent weight. After you find out the mass of the equivalent, divide it by the mass of the solution, which is indicated on the container with the solution or in the problem being solved. The answer will be the number of equivalents in the solution so that you can then calculate the normality. At the end of the answer, put the abbreviation "e."
3 Divide the actual weight of the solution by the equivalent weight. After you find out the mass of the equivalent, divide it by the mass of the solution, which is indicated on the container with the solution or in the problem being solved. The answer will be the number of equivalents in the solution so that you can then calculate the normality. At the end of the answer, put the abbreviation "e." - If in our example 75 g Ca (OH)2, then the formula will be as follows: 75 g ÷ 37 G.-e = 2.027 Oe.
 4 Divide the number of equivalents by the volume of solution in liters. Find out the total volume of the solution and write down the answer in liters. Take the number of equivalents just obtained and divide by the volume of the solution to find out the normality. At the end of your answer, put an abbreviation "N".
4 Divide the number of equivalents by the volume of solution in liters. Find out the total volume of the solution and write down the answer in liters. Take the number of equivalents just obtained and divide by the volume of the solution to find out the normality. At the end of your answer, put an abbreviation "N". - If there is 75 g of Ca (OH) in a solution with a volume of 8 liters2, then divide the number of equivalents by the volume in the following way: 2.027 Oe. ÷ 8 l = 0.253 N.
Tips
- Normal concentration, or normality, is commonly used to measure acids and bases. If you need to determine the concentration of another solution, the molarity is usually measured.
What do you need
- Periodic table
- Calculator