Author:
Monica Porter
Date Of Creation:
17 March 2021
Update Date:
1 July 2024
Content
Molar concentration indicates the relationship between the number of moles of a solute and the volume of the solution. To calculate molarity, you can start with moles and volume, mass and volume, or moles and milliliters (ml). Then, with the variables above, apply the basic molar concentration formula to get the correct result.
Steps
Method 1 of 4: Calculate Mol Concentration from Molar Number and Volume
It is important to know the basic formula for calculating the molar concentration. Molar concentration equal to the number of moles of a solute divided by the volume of the solution in liters. From there, we have the following formula: Molar concentration = number of moles of solute / number of liters of solution
- Example: What is the molar concentration of a solution containing 0.75 mol NaCl in 4.2 liters of solution?

Analyze the topic. To calculate the molar concentration, you need the number of moles and the volume of solution in liters. You do not need to calculate these two values because of the given topic.- For example:
- Number of moles = 0.75 moles of NaCl
- Volume = 4.2 L
- For example:
Divide the number of moles by the volume. The result of the mole division by volume is the number of moles per liter of solution, or molar concentration of that solution.
- Example: molar concentration = number of moles of solute / number of liters of solution = 0.75 mol / 4.2 L = 0.17857142

Record your results. Round to two or three numbers after the comma, depending on the teacher's request or the assignment. When recording your results, abbreviate "molar concentration" for "M" and accompany the solute's chemical symbol.- For example: 0.179 M NaCl
Method 2 of 4: Calculate Mol Concentration from Mass and Volume
It is necessary to understand the basic formula for calculating the molar concentration. Molar concentration shows the relationship between the number of moles of a solute and the volume of the solution. The formula for the molar concentration is as follows: molar concentration = solute concentration / number of liters of solution
- Problem example: Calculate the number of moles of the solution upon dissolving 3.4 g of KMnO4 in 5.2 liters of water.

Analyze the topic: To find the molar concentration, you need the number of moles and the volume of solution in liters. If these values are not given, but you know the volume and mass of the solution, you can determine the number of moles of solute before calculating the molar concentration.- For example:
- Weight = 3.4 g KMnO4
- Volume = 5.2 L
- For example:
Calculate the mass molecule of the solute. To calculate the number of moles of solute from that mass or grams of solute, you first need to determine the mass molecule of the solute. The mass molecule of a solute can be determined by adding the mass atom of each element in the solution. To find the cubic atom of each element, use the periodic table of elements.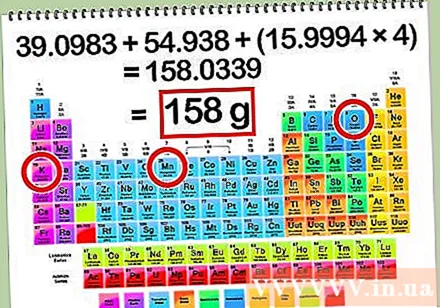
- For example:
- Mass atom of K = 39.1 g
- Mass atom of Mn = 54,9 g
- Mass atom of O = 16,0 g
- Total atoms of mass = K + Mn + O + O + O + O = 39.1 + 54.9 + 16 + 16 + 16 + 16 = 158.0 g
- For example:
Convert grams to moles. Once you have a cubic molecule, you need to multiply the number of grams of solute in the solution by the equivalent conversion factor of 1 mol per molar mass of the solute. The result of this multiplication is the number of moles of the solute.
- Example: grams of solute * (1 / molar mass of solute) = 3.4 g * (1 mol / 158 g) = 0.0215 mol
Divide the number of moles by the number of liters. Now that you have calculated the number of moles, now divide that number by the volume of the solution in liters, you will have the molar concentration of that solution.
- Example: molar concentration = number of moles of solute / number of liters of solution = 0.0215 mol / 5.2 L = 0.004134615
Record your results. You need to round up the results as required by the teacher, usually two to three numbers after a comma. In addition, when writing the result, abbreviate "molar concentration" as "M" and accompany the solute's chemical symbol.
- For example: 0.004 M KMnO4
Method 3 of 4: Calculate the Molar concentration from the Number of moles and Milliliters of solution
Need to know the formula for the molar concentration. To calculate the molar concentration. You need to calculate the number of moles of solute per liter of solution, not milliliters of solution. The general formula for calculating the molar concentration is: molar concentration = number of moles of solute / number of liters of solution
- Example: Calculate the molar concentration of a solution containing 1.2 moles of CaCl2 in 2905 milliliters of water.
Analyze the topic. To calculate the molar concentration, you need the number of moles of solute and the volume of solution in liters. If the volume of solution is given in the problem in milliliters, convert to the equivalent volume in liters before doing the calculation.
- For example:
- Number of moles = 1.2 moles of CaCl2
- Volume = 2905 ml
- For example:
Convert milliliters to liters. To convert the solution from milliliters to liters, divide the number of milliliters by 1000, because each liter equals 1000 milliliters. You can also convert milliliters to liters by shifting the decimal point 3 digits left.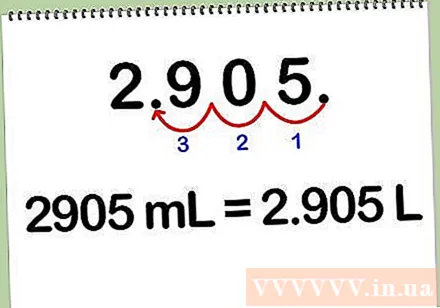
- For example: 2905 ml * (1 L / 1000 ml) = 2,905 L
Divide the number of moles by the number of liters. After you have the number of liters, you can calculate the molar concentration by dividing the number of moles by the number of liters of solution.
- Example: molar concentration = number of moles of solute / number of liters of solution = 1.2 moles of CaCl2 / 2,905 L = 0.413080895
Record your results. Remember to round the result to two or three commas, or as requested by your teacher. When recording the result, abbreviate "molar concentration" as "M" and then the chemical symbol for the solute.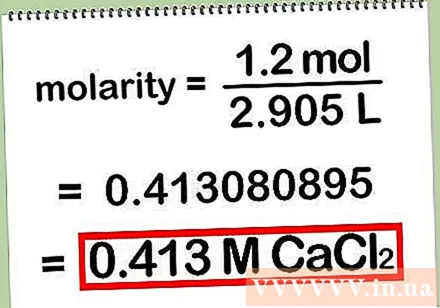
- For example: 0.413 M CaCl2
Method 4 of 4: Extra Practice
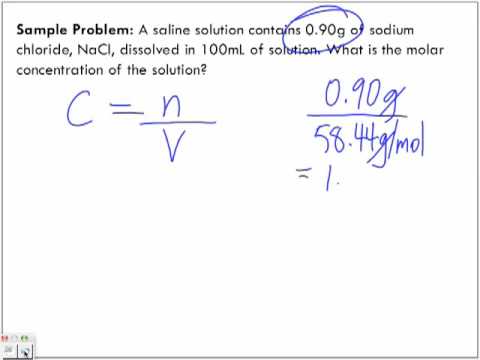
Calculate the molar concentration of a solution when 5.2 g NaCl is dissolved in 800 ml of water. Determine the values given by the problem: mass in grams and volume in milliliters.
- Mass = 5.2 g NaCl
- Volume = 800 ml of water
Find the mass molecule of NaCl by adding the cubic atom of the Na element, and the cubic atom of Cl.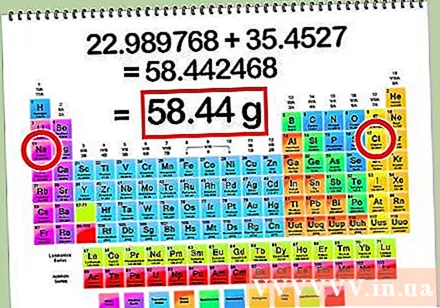
- Mass atom of Na = 22.99 g
- Mass atom of Cl = 35.45 g
- Mass molecules of NaCl = 22.99 + 35.45 = 58.44 g
Multiply the mass of the solute by the molar conversion factor. In this example, the molecular mass of NaCl is 58.44 g, so the conversion factor is “1 mol / 58.44 g”.
- Number of NaCl mole = 5.2 g NaCl * (1 mol / 58.44 g) = 0.8898 mol = 0.09 mol
Divide 800 ml of water by 1000, you will get the volume of water in liters.
- You can also multiply 800 ml by the 1 L / 1000 ml conversion factor from milliliter to liter.
- To shorten the multiplication process as above, you can back the decimal point 3 digits to the left.
- Volume = 800 ml * (1 L / 1000 ml) = 800 ml / 1000 ml = 0.8 L
Divide the number of moles of solute by the volume of solution in liters. To calculate the molar concentration, you need to divide 0.09 moles of solute (in this case, NaCl) by the volume of solution in liters.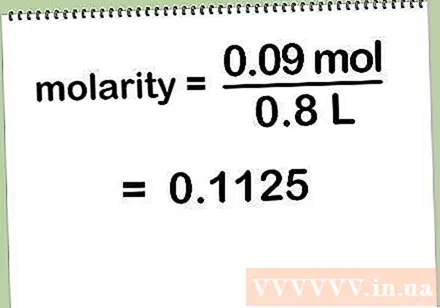
- Molar concentration = number of moles of solute / number of liters of solution = 0.09 mol / 0.8 L = 0.1125 mol / L
Record the final result. Round off the result to two or three numbers after the comma and abbreviate "molar concentration" with "M" together with the solute chemical symbol.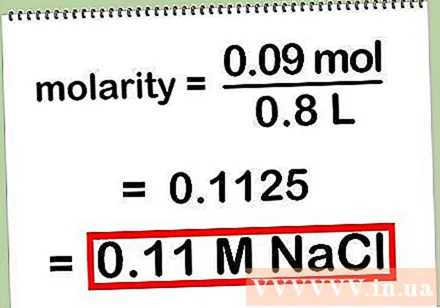
- Result: 0.11 M NaCl