Author:
Louise Ward
Date Of Creation:
11 February 2021
Update Date:
28 June 2024

Content
The size of an atom is so small that it is difficult to accurately measure the atomic number of a chemical compound. To be able to accurately measure a quantity of substances, scientists use a unit of mole to represent a specified number of atoms. One mole of substance is defined to be equivalent to the number of carbon atoms contained in 12 grams of carbon isotope, which is about 6,022 x 10 atoms. This value is called the Avogadro number, or the Avogadro constant. This is also referred to as the number of atoms in 1 mole of any element, and 1 mol of the mass of a substance is called the molar mass of that substance.
Steps
Method 1 of 2: Calculate the molar mass of an element
Definition of molar mass. Molar mass of a substance is the mass (in grams) of one mole of that substance. To calculate the molar mass of an element, multiply its atomic mass by the conversion factor grams per mol (g / mol).
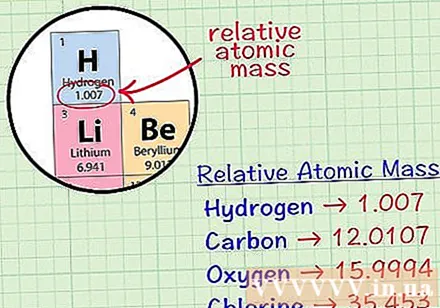
Find the average cubic atom of an element. The average mass atom of an element is the average mass, in atomic units, in a sample that includes all of the isotopes of that element. This information is often given on the periodic table of elements. By locating an element, you can find an average cubic atom written just below the element's chemical symbol. This value is not an integer, but a number with decimals.- For example, with hydrogen, the average mass atom is 1.007; The average cubic atom of carbon is 12,0107; The average mass atom of oxygen is 15,9994; chlorine has an average atomic mass of 35,453.

Multiply the average mass atom by the molar mass constant. The unit of molar glide is defined as 0.001 kilogram per mol, or 1 gram per mol. The product of the average mass atom and the molar mass constant converts the unit of atomic mass to grams per mole, so the molar mass of hydrogen will be 1.007 grams per mol, for carbon is 12. , 0107 grams per mole, of oxygen is 15,9995 grams per mole, and that of chlorine is 35,453 grams per mole.- Some elements exist in nature as molecules consisting of two or more of the same atoms. That is, if you want to calculate the molar mass of compounds made up of more than one atom, such as hydrogen gas, oxygen gas or chlorine gas, you need to determine the average atomic mass of the compound and multiply this value. with the molar mass constant, '' then '' multiply the product you just found by 2.
- With H2: 1,007 x 2 = 2,014 grams per mol; for O2: 15,9994 x 2 = 31,9988 grams per mol; and Cl2: 35,453 x 2 = 70,096 grams per mol.
Method 2 of 2: Calculate the molar mass of the compound

Determine the structural formula of the compound. The structural formula of a substance gives the atomic number of each element that makes up that compound. (This information is available in all reference books). For example, the chemical formula of hydrochloric acid is HCl; of glucose is C6H12O6. With this structural formula, we can determine the number of each type of atom constituting the compound under consideration.- Where HCl has one hydrogen atom and one chlorine atom.
- Glucose sugar molecule C6H12O6 has 6 carbon atoms, 12 hydrogen atoms and 6 oxygen atoms.
Determine the average mass atom of each constituent element. Use the periodic table to find the average mass atom of each element present in the compound. The average mass atom is usually written under the element's chemical symbol on the periodic table. Similar to calculating the molar mass of an element, multiply the average mass atom by 1 gram / mol.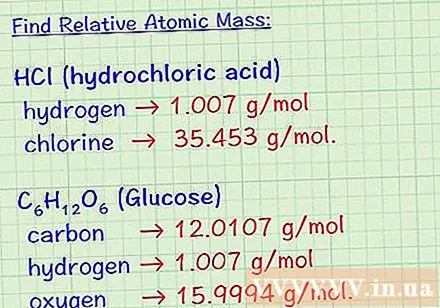
- The average mass atom of the elements forming hydrochloric acid is as follows: hydrogen 1,007 g / mol and chlorine 35,453 g / mol.
- The average mass atom of the elements that make up the glucose molecule is: carbon, 12,017 g / mol; hydrogen, 1,007 g / mol; and oxygen, 15,995 g / mol.
Calculate the molar mass of each element. Multiplying an element's mass atom by the number of atoms it contributes in a compound gives the average mass of the element in the compound.
- In the case of hydrochloric acid, HCl, the molar mass of the element hydrogen is 1,007 g / mol, and that of chlorine is 35,453 g / mol.
- In the case of glucose, C6H12O6, the molar mass of each element is as follows: carbon, 12,0107 x 6 = 72,0642 g / mol; hydrogen, 1,007 x 12 = 12,084 g / mol; oxygen, 15,9995 x 6 = 95,9964 g / mol.
Total molar mass of the constituent elements. The total molar mass of the composting elements is the molar mass of the compound.In the previous step we calculated the molar mass of each element present in the compound, in this step we just need to add all these values together.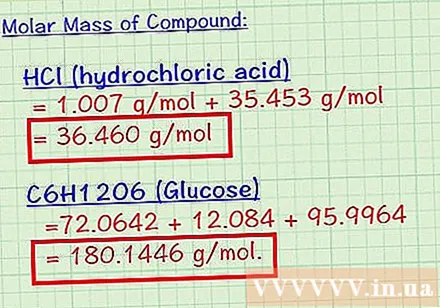
- Molar mass of hydrochloric acid is 1,007 + 35,453 = 36,460 g / mol. 36.46 grams is the mass of 1 mol hydrochloric acid.
- Molar mass of glucose is 72,0642 + 12,084 + 95,9964 = 180,1446 g / mol. So each mol of glucose has a mass of 180.14 grams.
Advice
- Although in most cases the average mass atom is recorded to 1 part 1000 (4 decimal places), in laboratories, the molar mass is often reduced to 2 decimal places, sometimes even less, for large molecules. Therefore, in the laboratory case, the molar mass of hydrochloric acid may be written as 36.46 grams per mol, for glucose 180.14 grams per mol.
What you need
- Chemical reference book or periodic table of elements
- Computer