Author:
Robert Simon
Date Of Creation:
19 June 2021
Update Date:
1 July 2024
Content
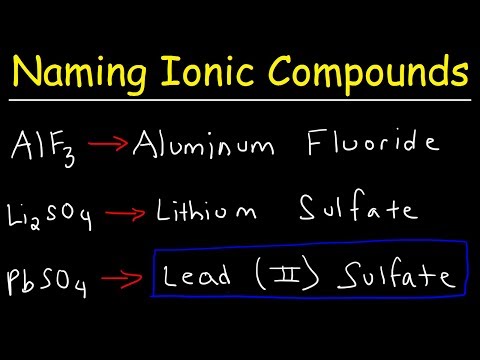
Ionic compounds are made up of cations (positive ions) and anions (negative ions). Ionic compounds usually consist of a metallic element and one or more non-metallic elements. To name an ionic compound, you need to find the names of the cations and the anions that make up that compound. First write the name of the metal, followed by the name of the corresponding non-metallic base. If you want to know how to name an ionic compound in any case, follow these steps.
Steps
Method 1 of 3: Basic ionic compound
Write down the chemical formula of the ionic compound. Suppose we have ionic compounds NaCl.

Write down the name of the metal or cation. This is the ion with a positive charge in a compound, and it is always written first in a compound's chemical formula. Na is sodium, so write Sodium.
Write down the name of the nonmetal or anion. Add "-ua" after the element name (if the element name ends in O, add r before ua for easy reading). Cl is chlorine, add "rua" at the end so it will read as chloride.

Combine names. NaCl can be written as sodium chloride.
Practice naming simple ionic compounds. Once you understand this designation, try naming a few simple ionic compounds. Memorizing a few examples will also help you better understand how ionic compounds are called. Here are some compounds:
- Li2S = Lithium sulfide

- Ag2S = Silver sulfide

- MgCl2 = Magnesium chloride
- Li2S = Lithium sulfide
Method 2 of 3: Transition metal
Write down the chemical formula of the ionic compound. You can find transition metals in the middle of the periodic table. They are called transition metals because their oxidation numbers or charges change continuously. Suppose we have the following compound: Fe2O3.
- Determine the charge of metals. Since metals have a positive charge, you take the number 3 from O3 put up and then cross Fe has +3 charge (if you want, you can do the opposite and write O has charge -2). Sometimes people will give you an electrical charge.
Write down the name of the metal. You know Fe is iron and has a charge of +3, so it can be called Iron (III). Remember to use Roman numerals when writing names, and when writing chemical formulas, do not use Roman numerals.
Write down the name of nonmetals. You know O is oxygen, add "t" to the end and we have "oxide".
Place the first and second names side by side. Now we have the name of the compound. Fe2O3 = Iron (III) oxide.
Use old naming conventions. When consulting English books, you may encounter old names. In the old naming conventions, you used the "-ous" and "-ic" ends when using metal names instead of Roman numerals. If iron metal has a lower oxidation number (less than a unit of charge, regardless of "+" or "-"), add the "-ous" tail. If it has a higher oxidation number then add the "-ic" extension. Fe has a lower oxidation number (Fe has a higher oxidation number), so we call it ferrous. Thus the name of the FeO compound is ferrous oxide.
Remember the exceptions. There are two constant charged transition metals zinc (Zn) and silver (Ag). This means that you do not need to use Roman numerals to name these elements. advertisement
Method 3 of 3: Compound with polyatomic ions
Write down the formula for polyatomic ions. This compound is made up of more than two ions. Suppose we have the following compound: FeNH4(SO4)2.
Find metal charges. You have to do some math to figure it out. First, you know sulfate ions or SO4 has a charge of -2, and there are two ions because there is a 2 below the parentheses. We have 2 x -2 = -4. Next you know NH4, or the ammonium ion, has a charge of +1. Adding -4 to 1 gives -3. That is, Fe ions must have a charge of +3 for the compound to be electrically neutral.
Write down the name of the metal. In this case you can write as Iron (III).
Name the non-metallic ion. In the above example, the names of the two ions are respectively ammonium and sulfate, or collectively amonisunfat.
Combine metal names and nonmetal ions names. You can call the name of the FeNH compound4(SO4)2 was iron (III) amonisunfat. advertisement
Two-component compounds are essentially ionic compounds that either gain or lose electrons depending on the oxidation state.
Advice
- When you have the name of a compound and want to write its chemical formula (already with a Roman numeral), we take the charge of the positive ion diagonally down to get the number of nonmetallic radical molecules. The Roman numeral is the charge of the positive ion.