Author:
Randy Alexander
Date Of Creation:
2 April 2021
Update Date:
1 July 2024

Content
A chemical equation is a symbolic representation of a chemical reaction. The reagents are written on the left-hand side and the product on the right-hand side. The law of conservation of mass indicates that no atoms are born or lost in a chemical reaction, so the number of atoms present in the reactant must be equal to the number of atoms present in the reaction product. Following this tutorial, you can balance chemical equations in different ways.
Steps
Method 1 of 2: Balance according to the traditional method
Write the given equation. In this example, you would have: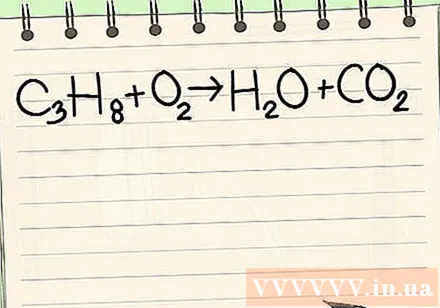
- C3H8 + O2 -> H2O + CO2
- This reaction occurs when propane (C3H8burned in oxygen to form water and carbon dioxide.
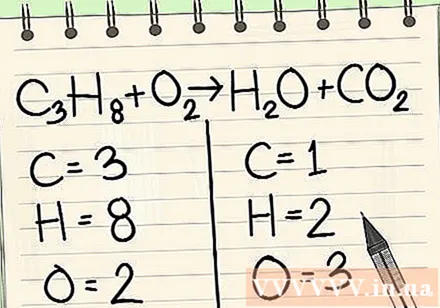
Write down the number of atoms for each element you have on each side of the equation. See the indexes below next to each atom to find the number of atoms in the equation.- Left: 3 carbon, 8 hydrogen and 2 oxygen.
- Right: 1 carbon, 2 hydrogen and 3 oxygen.
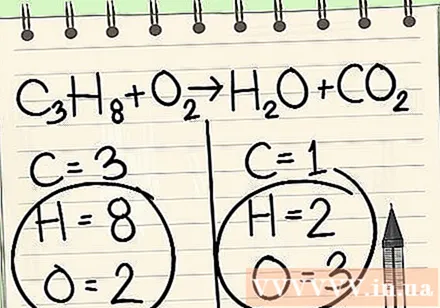
Always leave hydrogen and oxygen in the end.
If you have more than one element left to balance: Select an element that appears only in the single molecule of the reactant and only in the single molecule of the product. This means that you will need to balance the carbon atoms first.
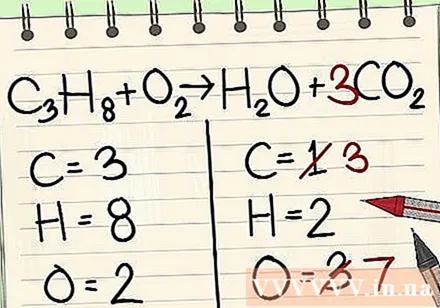
Add the coefficient for single carbon atoms to the right side of the equation to balance it with the three carbon atoms on the left side of the equation.- C3H8 + O2 -> H2O + 3CO2
- A factor of 3 in front of carbon on the right side indicates there are 3 carbon atoms as a sub-3 on the left indicates 3 carbon atoms.
- In a chemical equation, you can change the coefficient, but not the subscript.
Next is the hydrogen atomic balance. You have 8 hydrogen atoms on the left. Hence you will need 8 on the right side.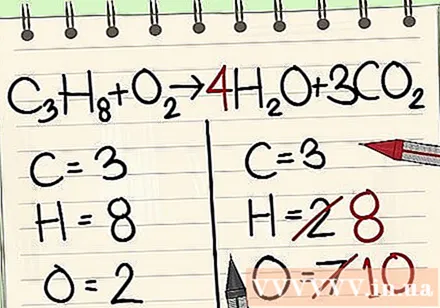
- C3H8 + O2 -> 4H2O + 3CO2
- To the right of the hour you add 4 as the factor because the bottom number shows you already have 2 hydrogen atoms.
- When you multiply factor 4 by index 2, you get 8.
- The other 6 oxygen atoms are from 3CO2. (3x2 = 6 oxygen atoms + 4 other oxygen atoms = 10)
Balance oxygen atoms.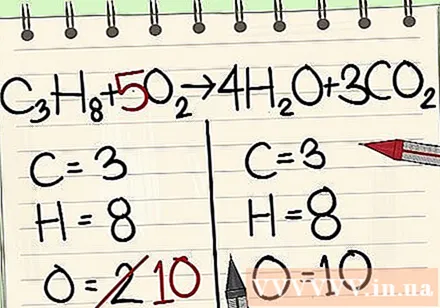
- Because you've added coefficients to the molecules to the right of the equation, the number of oxygen atoms has changed. Now you have 4 oxygen atoms in the water molecule and 6 oxygen atoms in the carbon dioxide molecule. In total we have 10 oxygen atoms.
- Add the factor 5 to the oxygen molecule to the left of the equation. Now you have 10 oxygen molecules on each side.
- C3H8 + 5O2 -> 4H2O + 3CO2.

- The carbon, hydrogen, and oxygen atoms are in balance. Your equation is complete.
Method 2 of 2: Balance according to the algebraic method
Write equations according to symbols and formulas. Example a = 1 and write the equation based on that formula.
Replace digits with their variables.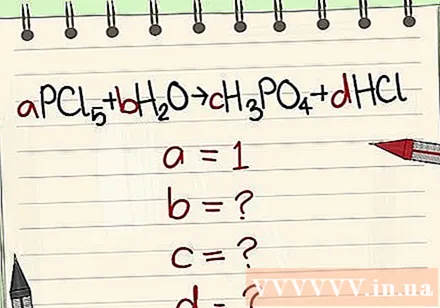
Check the amount of elements in the reaction side as well as the product side.
- Example: aPCl5 + bH2O = cH3PO4 + dHCl so that a = 1 b = c = d = and separating the elements P, Cl, H, O, so you get a = 1 b = 4 c = 1 d = 5 .
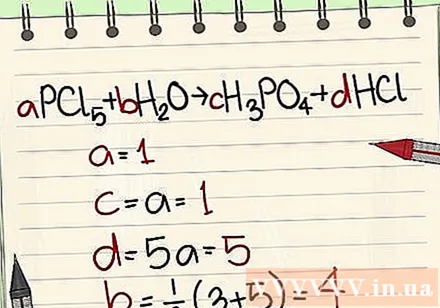
- Example: aPCl5 + bH2O = cH3PO4 + dHCl so that a = 1 b = c = d = and separating the elements P, Cl, H, O, so you get a = 1 b = 4 c = 1 d = 5 .
Advice
- Remember to simplify the equation.
- If you have trouble, you can type an equation into the online balance tool to balance it out. Remember when you take the exam you don't have access to an online balance, so don't depend on it.
Warning
- Never use a coefficient as a fraction in a chemical equation - you cannot divide molecules or atoms in a chemical reaction.
- During the equilibrium process, you can use fractions but the equation will not be balanced if the coefficients are still fractions.
- To remove fractions, multiply the entire equation (both left and right) by the denominator of the fraction.