Author:
Christy White
Date Of Creation:
4 May 2021
Update Date:
1 July 2024

Content
Whenever you mix chemical components, whether in the kitchen or in a chemical laboratory, you create new substances, what we call “products”. During these chemical reactions, heat can be absorbed from or given up to the environment. The exchange of heat during a chemical reaction with the environment is known as the enthalpy of a reaction, written as ∆H. To find ∆H, read the following article.
To step
 Prepare the reactants for the chemical reaction. In order to correctly measure the enthalpy of a reaction, you will first need to have the correct amount of each reactant.
Prepare the reactants for the chemical reaction. In order to correctly measure the enthalpy of a reaction, you will first need to have the correct amount of each reactant. - Suppose, as an example, that you want to find the enthalpy of the reaction in which water is formed from hydrogen and oxygen: 2H2 (Hydrogen) + O2 (Oxygen) → 2H2O (Water). For the purposes of this example, assume we have 2 moles of hydrogen and 1 mole of oxygen.
 Clean the reaction vessel. To make sure the reaction takes place without contamination, clean the reaction vessel (usually a calorimeter) that you want to use.
Clean the reaction vessel. To make sure the reaction takes place without contamination, clean the reaction vessel (usually a calorimeter) that you want to use.  Place a stir stick and thermometer in the reaction vessel. Prepare the mixture as needed and measure their temperature by holding both the stir stick and thermometer in the calorimeter.
Place a stir stick and thermometer in the reaction vessel. Prepare the mixture as needed and measure their temperature by holding both the stir stick and thermometer in the calorimeter.  Pour the reactants into the reaction vessel. Once everything is properly prepared, you can put the reactants in the calorimeter. Then close it immediately.
Pour the reactants into the reaction vessel. Once everything is properly prepared, you can put the reactants in the calorimeter. Then close it immediately.  Measure the temperature. Using the thermometer that you placed in the calorimeter, immediately record the temperature after adding the reactants.
Measure the temperature. Using the thermometer that you placed in the calorimeter, immediately record the temperature after adding the reactants. - In the example above, suppose you put hydrogen and oxygen in the calorimeter, shut it off, and noted a temperature (T1) of 150K (which is very low).
 Continue with the response. Give the substances some time to react, stir if necessary to speed it up exactly.
Continue with the response. Give the substances some time to react, stir if necessary to speed it up exactly.  Measure the temperature again. When the reaction is complete, record the temperature again.
Measure the temperature again. When the reaction is complete, record the temperature again. - Suppose in the example the second temperature is (T2) or 95K.
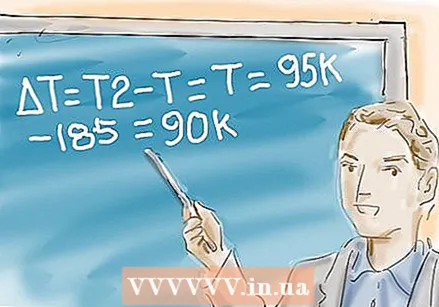 Calculate the difference in temperature of T1 and T. You note the difference as ∆T.
Calculate the difference in temperature of T1 and T. You note the difference as ∆T. - In the example you calculate ∆T as follows:
∆T = T2 - T1 = 95K - 185K = -90K
- In the example you calculate ∆T as follows:
 Determine the total mass of the reactants. If you want to calculate the total mass of the reactants, you need the molar mass of your components. Molar mass is a constant; you can find these in standard periodic tables or other chemistry tables.
Determine the total mass of the reactants. If you want to calculate the total mass of the reactants, you need the molar mass of your components. Molar mass is a constant; you can find these in standard periodic tables or other chemistry tables. - In the example above, you use hydrogen and oxygen, which have molar masses of 2g and 32g respectively. Since you have 2 moles of hydrogen and used 1 mole of oxygen, you can calculate the total mass of the reactants as follows:
2x (2g) + 1x (32g) = 4g + 32g = 36g
- In the example above, you use hydrogen and oxygen, which have molar masses of 2g and 32g respectively. Since you have 2 moles of hydrogen and used 1 mole of oxygen, you can calculate the total mass of the reactants as follows:
 Calculate the enthalpy of the reaction. Once you have done this you can determine the enthalpy of the reaction. The formula looks like this: ∆H = (m) x (s) x (∆T)
Calculate the enthalpy of the reaction. Once you have done this you can determine the enthalpy of the reaction. The formula looks like this: ∆H = (m) x (s) x (∆T) - In the formula, m is the total mass of the reactants; s is the specific heat, which is also constant for each element or compound material.
- In the example above, the final product is water, with a specific heat of 4.2 JK-1 g-1. The enthalpy of the reaction can therefore be calculated as follows:
∆H = (36g) x (4.2 JK-1 g-1) x (-90K) = -13608 J
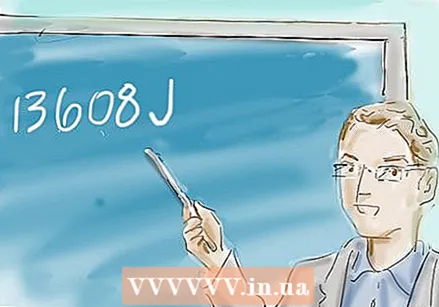 Make a note of the result. If the sign of your answer is negative, then the reaction is exothermic: heat is lost to the environment. If the sign of the answer is positive, then the reaction is endothermic: heat is absorbed from the environment.
Make a note of the result. If the sign of your answer is negative, then the reaction is exothermic: heat is lost to the environment. If the sign of the answer is positive, then the reaction is endothermic: heat is absorbed from the environment. - In the example above, the last answer is -13608 J. So this is an exothermic reaction that uses a significant amount of energy.
Tips
- These calculations are done in Kelvin (K) - a scale for the temperature measurement just like Celsius. If you want to convert Kelvin to Celsius, just add 273 degrees: K = C + 273.