Author:
Ellen Moore
Date Of Creation:
20 January 2021
Update Date:
1 July 2024

Content
- Steps
- Method 1 of 3: Using the Clapeyron-Clausius equation
- Method 2 of 3: Calculating vapor pressure in solutions
- Method 3 of 3: Calculating Steam Pressure in Special Cases
- Tips
Have you ever left a bottle of water for several hours under the scorching sun and heard a “hissing” sound when you open it? This sound is caused by steam pressure. In chemistry, vapor pressure is the pressure exerted by the vapor of a liquid that evaporates in a hermetically sealed container. To find the vapor pressure at a given temperature, use the Clapeyron-Clausius equation: ln (P1 / P2) = (ΔHvap/ R) ((1 / T2) - (1 / T1)).
Steps
Method 1 of 3: Using the Clapeyron-Clausius equation
 1 Write down the Clapeyron-Clausius equation that is used to calculate vapor pressure as it changes over time. This formula can be used for most physical and chemical problems. The equation looks like this: ln (P1 / P2) = (ΔHvap/ R) ((1 / T2) - (1 / T1)), where:
1 Write down the Clapeyron-Clausius equation that is used to calculate vapor pressure as it changes over time. This formula can be used for most physical and chemical problems. The equation looks like this: ln (P1 / P2) = (ΔHvap/ R) ((1 / T2) - (1 / T1)), where: - ΔHvap Is the enthalpy of vaporization of the liquid. It can usually be found in a table in chemistry textbooks.
- R - gas constant equal to 8.314 J / (K × mol)
- T1 is the initial temperature (at which the vapor pressure is known).
- T2 is the final temperature (at which the vapor pressure is unknown).
- P1 and P2 - steam pressure at temperatures T1 and T2, respectively.
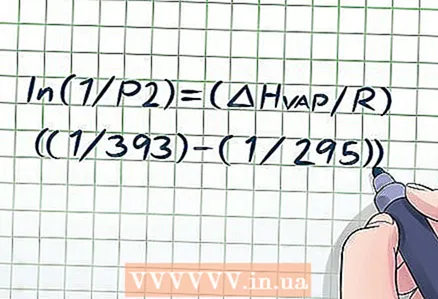 2 Substitute the values of quantities given to you into the Clapeyron-Clausius equation. Most problems give two temperature values and a pressure value, or two pressure values and a temperature value.
2 Substitute the values of quantities given to you into the Clapeyron-Clausius equation. Most problems give two temperature values and a pressure value, or two pressure values and a temperature value. - For example, a vessel contains a liquid at a temperature of 295 K, and its vapor pressure is 1 atmosphere (1 atm). Find the vapor pressure at 393 K. Here you are given two temperatures and a pressure, so you can find a different pressure using the Clapeyron-Clausius equation. Substituting the values given to you in the formula, you get: ln (1 / P2) = (ΔHvap/ R) ((1/393) - (1/295)).
- Please note that in the Clapeyron-Clausius equation, temperature is always measured in kelvin, and pressure in any unit of measure (but they must be the same for P1 and P2).
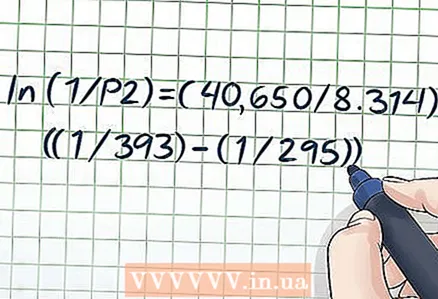 3 Substitute the constants. The Clapeyron-Clausius equation contains two constants: R and ΔHvap... R is always 8.314 J / (K × mol). ΔH valuevap (enthalpy of vaporization) depends on the substance, the vapor pressure of which you are trying to find; this constant can usually be found in a table in chemistry textbooks or on websites (for example, here).
3 Substitute the constants. The Clapeyron-Clausius equation contains two constants: R and ΔHvap... R is always 8.314 J / (K × mol). ΔH valuevap (enthalpy of vaporization) depends on the substance, the vapor pressure of which you are trying to find; this constant can usually be found in a table in chemistry textbooks or on websites (for example, here). - In our example, let's say that there is water in the vessel. ΔHvap water is equal to 40.65 kJ / mol or equal to 40650 J / mol.
- Plug the constants into the formula and get: ln (1 / P2) = (40650/8314) ((1/393) - (1/295)).
 4 Solve the equation using algebraic operations.
4 Solve the equation using algebraic operations.- In our example, the unknown variable is under the sign of the natural logarithm (ln). To get rid of the natural logarithm, convert both sides of the equation to the power of the mathematical constant "e". In other words, ln (x) = 2 → e = e → x = e.
- Now solve the equation:
- ln (1 / P2) = (40650 / 8.314) ((1/393) - (1/295))
- ln (1 / P2) = (4889.34) (- 0.00084)
- (1 / P2) = e
- 1 / P2 = 0.0165
- P2 = 0.0165 = 60.76 atm. This makes sense, as raising the temperature in a hermetically sealed container by 100 degrees will increase vaporization, which will significantly increase the vapor pressure.
Method 2 of 3: Calculating vapor pressure in solutions
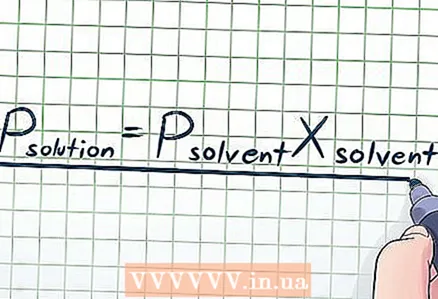 1 Write down Raoult's law. In real life, pure liquids are rare; we often deal with solutions. A solution is made by adding a small amount of a certain chemical called a "solute" to a larger amount of another chemical called a "solvent." In the case of solutions, use Raoult's law:Psolution = PsolventXsolvent, where:
1 Write down Raoult's law. In real life, pure liquids are rare; we often deal with solutions. A solution is made by adding a small amount of a certain chemical called a "solute" to a larger amount of another chemical called a "solvent." In the case of solutions, use Raoult's law:Psolution = PsolventXsolvent, where: - Psolution Is the vapor pressure of the solution.
- Psolvent Is the vapor pressure of the solvent.
- Xsolvent - the mole fraction of the solvent.
- If you don't know what a mole fraction is, read on.
 2 Determine which substance will be the solvent and which will be the solute. Recall that a solute is a substance that dissolves in a solvent, and a solvent is a substance that dissolves a solute.
2 Determine which substance will be the solvent and which will be the solute. Recall that a solute is a substance that dissolves in a solvent, and a solvent is a substance that dissolves a solute. - Consider a syrup example. To obtain a syrup, one part of sugar is dissolved in one part of water, so sugar is a solute and water is a solvent.
- Note that the chemical formula for sucrose (common sugar) is C12H22O11... We will need it in the future.
 3 Find the temperature of the solution, as it will affect its vapor pressure. The higher the temperature, the higher the vapor pressure, since vaporization increases with increasing temperature.
3 Find the temperature of the solution, as it will affect its vapor pressure. The higher the temperature, the higher the vapor pressure, since vaporization increases with increasing temperature. - In our example, let's say the syrup temperature is 298 K (about 25 ° C).
 4 Find the vapor pressure of the solvent. Vapor pressure values for many common chemicals are given in chemistry handbooks, but these values are usually given at temperatures of 25 ° C / 298 K or at their boiling points. If in the problem you are given such temperatures, use the values from the reference books; otherwise, you need to calculate the vapor pressure at a given temperature of the substance.
4 Find the vapor pressure of the solvent. Vapor pressure values for many common chemicals are given in chemistry handbooks, but these values are usually given at temperatures of 25 ° C / 298 K or at their boiling points. If in the problem you are given such temperatures, use the values from the reference books; otherwise, you need to calculate the vapor pressure at a given temperature of the substance. - To do this, use the Clapeyron-Clausius equation, substituting the vapor pressure and temperature of 298 K (25 ° C) instead of P1 and T1, respectively.
- In our example, the temperature of the solution is 25 ° C, so use the value from the reference tables - the vapor pressure of water at 25 ° C is 23.8 mmHg.
 5 Find the mole fraction of the solvent. To do this, find the ratio of the number of moles of a substance to the total number of moles of all substances in the solution. In other words, the mole fraction of each substance is (number of moles of the substance) / (the total number of moles of all substances).
5 Find the mole fraction of the solvent. To do this, find the ratio of the number of moles of a substance to the total number of moles of all substances in the solution. In other words, the mole fraction of each substance is (number of moles of the substance) / (the total number of moles of all substances). - Let's say that you used 1 liter of water and 1 liter of sucrose (sugar) to make a syrup. In this case, it is necessary to find the number of moles of each substance. To do this, you need to find the mass of each substance, and then use the molar masses of these substances to get moles.
- Weight of 1 liter of water = 1000 g
- Weight of 1 liter of sugar = 1056.7 g
- Mole (water): 1000 g × 1 mol / 18.015 g = 55.51 mol
- Mole (sucrose): 1056.7 g × 1 mol / 342.2965 g = 3.08 mol (note that you can find the molar mass of sucrose from its chemical formula C12H22O11).
- Total number of moles: 55.51 + 3.08 = 58.59 mol
- Mole fraction of water: 55.51 / 58.59 = 0.947.
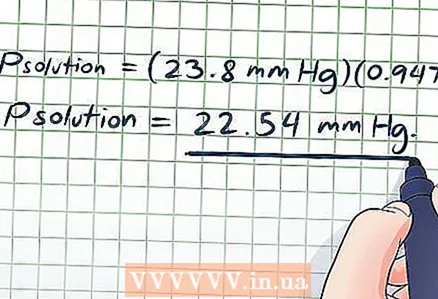 6 Now plug the data and the found values u200b u200bof the quantities into the Raoult equation given at the beginning of this section (Psolution = PsolventXsolvent).
6 Now plug the data and the found values u200b u200bof the quantities into the Raoult equation given at the beginning of this section (Psolution = PsolventXsolvent).- In our example:
- Psolution = (23.8 mmHg) (0.947)
- Psolution = 22.54 mmHg Art. This makes sense, since a small amount of sugar is dissolved in a large amount of water (if measured in moles; their amount is the same in liters), so the vapor pressure will decrease slightly.
Method 3 of 3: Calculating Steam Pressure in Special Cases
 1 Definition of standard conditions. Often in chemistry, temperature and pressure values are used as a kind of "default" value. These values are called standard temperature and pressure (or standard conditions). In steam pressure problems, standard conditions are often mentioned, so it is better to remember the standard values:
1 Definition of standard conditions. Often in chemistry, temperature and pressure values are used as a kind of "default" value. These values are called standard temperature and pressure (or standard conditions). In steam pressure problems, standard conditions are often mentioned, so it is better to remember the standard values: - Temperature: 273.15 K / 0˚C / 32 F
- Pressure: 760 mmHg / 1 atm / 101.325 kPa
 2 Rewrite the Clapeyron-Clausius equation to find other variables. The first section of this article showed how to calculate the vapor pressures of pure substances. However, not all problems require finding the pressure P1 or P2; in many problems it is necessary to calculate the temperature or the value of ΔHvap... In such cases, rewrite the Clapeyron-Clausius equation by isolating the unknown on one side of the equation.
2 Rewrite the Clapeyron-Clausius equation to find other variables. The first section of this article showed how to calculate the vapor pressures of pure substances. However, not all problems require finding the pressure P1 or P2; in many problems it is necessary to calculate the temperature or the value of ΔHvap... In such cases, rewrite the Clapeyron-Clausius equation by isolating the unknown on one side of the equation. - For example, given an unknown liquid, the vapor pressure of which is 25 Torr at 273 K and 150 Torr at 325 K. It is necessary to find the enthalpy of vaporization of this liquid (that is, ΔHvap). The solution to this problem:
- ln (P1 / P2) = (ΔHvap/ R) ((1 / T2) - (1 / T1))
- (ln (P1 / P2)) / ((1 / T2) - (1 / T1)) = (ΔHvap/ R)
- R × (ln (P1 / P2)) / ((1 / T2) - (1 / T1)) = ΔHvap Now substitute the given values for you:
- 8.314 J / (K × mol) × (-1.79) / (- 0.00059) = ΔHvap
- 8.314 J / (K × mol) × 3033.90 = ΔHvap = 25223.83 J / mol
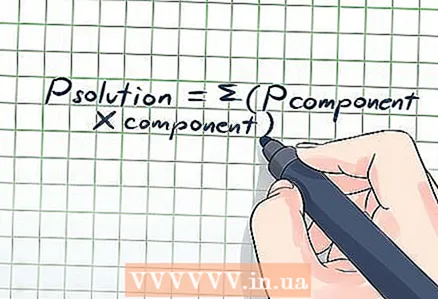 3 Consider the vapor pressure of the permeate. In our example from the second section of this article, the solute - sugar - does not evaporate, but if the solute produces steam (evaporates), the vapor pressure should be taken into account. To do this, use a modified form of Raoult's equation: Psolution = Σ (PsubstanceXsubstance), where the symbol Σ (sigma) means that it is necessary to add the values of the vapor pressures of all substances that make up the solution.
3 Consider the vapor pressure of the permeate. In our example from the second section of this article, the solute - sugar - does not evaporate, but if the solute produces steam (evaporates), the vapor pressure should be taken into account. To do this, use a modified form of Raoult's equation: Psolution = Σ (PsubstanceXsubstance), where the symbol Σ (sigma) means that it is necessary to add the values of the vapor pressures of all substances that make up the solution. - For example, consider a solution made of two chemicals: benzene and toluene. The total volume of the solution is 120 milliliters (ml); 60 ml of benzene and 60 ml of toluene.The solution temperature is 25 ° C, and the vapor pressure at 25 ° C is 95.1 mm Hg. for benzene and 28.4 mm Hg. for toluene. It is necessary to calculate the vapor pressure of the solution. We can do this using the densities of substances, their molecular weights and vapor pressure values:
- Weight (benzene): 60 ml = 0.06 l × 876.50 kg / 1000 l = 0.053 kg = 53 g
- Mass (toluene): 0.06 L × 866.90 kg / 1000 L = 0.052 kg = 52 g
- Mole (benzene): 53 g × 1 mol / 78.11 g = 0.679 mol
- Mole (toluene): 52 g × 1 mol / 92.14 g = 0.564 mol
- Total number of moles: 0.679 + 0.564 = 1.243
- Mole fraction (benzene): 0.679 / 1.243 = 0.546
- Mole fraction (toluene): 0.564 / 1.243 = 0.454
- Solution: Psolution = PbenzeneXbenzene + PtolueneXtoluene
- Psolution = (95.1 mmHg) (0.546) + (28.4 mmHg) (0.454)
- Psolution = 51.92 mm Hg. Art. + 12.89 mm Hg. Art. = 64.81 mmHg Art.
Tips
- To use the Clapeyron Clausius equation, the temperature must be specified in degrees Kelvin (denoted by K). If your temperature is given in Celsius, you need to convert it using the following formula: Tk = 273 + Tc
- The above method works because energy is directly proportional to the amount of heat. The temperature of the liquid is the only environmental factor that affects the vapor pressure.