Author:
Clyde Lopez
Date Of Creation:
26 June 2021
Update Date:
1 July 2024

Content
- Steps
- Method 1 of 4: Calculating Percentage Using a Weight / Volume Formula
- Method 2 of 4: Preparation of a molecular solution
- Method 3 of 4: Diluting Solutions of Known Concentration
- Method 4 of 4: Compliance with safety precautions
- Tips
- Warnings
- What do you need
Simple chemical solutions can be easily prepared in a variety of ways at home or at work. Whether you are making a solution from a powdery material or diluting a liquid, it is easy to determine the correct amount of each component. When preparing chemical solutions, remember to use personal protective equipment to avoid damage.
Steps
Method 1 of 4: Calculating Percentage Using a Weight / Volume Formula
 1 Define percentage content on weight/ volume of solution. Percentages show how many parts of a substance are in one hundred parts of a solution. When applied to chemical solutions, this means that if the concentration is 1 percent, then 100 milliliters of solution contains 1 gram of the substance, that is, 1 ml / 100 ml.
1 Define percentage content on weight/ volume of solution. Percentages show how many parts of a substance are in one hundred parts of a solution. When applied to chemical solutions, this means that if the concentration is 1 percent, then 100 milliliters of solution contains 1 gram of the substance, that is, 1 ml / 100 ml. - For example, by weight: A 10% solution by weight contains 10 grams of the substance dissolved in 100 milliliters of solution.
- For example, by volume: A 23 percent solution by volume contains 23 milliliters of liquid compound in every 100 milliliters of solution.
 2 Determine the volume of the solution you want to prepare. To find out the required mass of a substance, you must first determine the final volume of the solution you need. This volume depends on how much solution you need, how often you will use it, and the stability of the finished solution.
2 Determine the volume of the solution you want to prepare. To find out the required mass of a substance, you must first determine the final volume of the solution you need. This volume depends on how much solution you need, how often you will use it, and the stability of the finished solution. - If you need to use a fresh solution each time, prepare only the amount you need for one use.
- If the solution retains its properties for a long time, you can prepare a larger amount for use in the future.
- Example: You need to prepare a 5% NaCl solution with a volume of 500 ml.
 3 Calculate the number of grams of the substance that is required to prepare the solution. To calculate the required number of grams, use the following formula: number of grams = (percentage required) (required volume / 100 ml). In this case, the required percentages are expressed in grams, and the required volume in milliliters.
3 Calculate the number of grams of the substance that is required to prepare the solution. To calculate the required number of grams, use the following formula: number of grams = (percentage required) (required volume / 100 ml). In this case, the required percentages are expressed in grams, and the required volume in milliliters. - Example: You need to prepare a 5% NaCl solution with a volume of 500 ml.
- number of grams = (5g) (500ml / 100ml) = 25 grams.
- If NaCl is given as a solution, simply take 25 milliliters of NaCl instead of grams of powder and subtract that volume from the final volume: 25 milliliters of NaCl to 475 milliliters of water.
 4 Weigh the substance. After you calculate the required mass of the substance, you should measure this amount. Take a calibrated scale, place a bowl on it and set zero. Weigh the required amount of substance in grams and pour it out.
4 Weigh the substance. After you calculate the required mass of the substance, you should measure this amount. Take a calibrated scale, place a bowl on it and set zero. Weigh the required amount of substance in grams and pour it out. - Before continuing to prepare the solution, be sure to clean the weighing pan of any powder residues.
- In the example above, you need to weigh 25 grams of NaCl.
 5 Dissolve the substance in the required amount of liquid. Unless otherwise specified, water is used as the solvent. Take a measuring glass and measure out the required amount of liquid. Then dissolve the powder material in the liquid.
5 Dissolve the substance in the required amount of liquid. Unless otherwise specified, water is used as the solvent. Take a measuring glass and measure out the required amount of liquid. Then dissolve the powder material in the liquid. - Sign the container in which you will store the solution. Clearly indicate the substance and its concentration on it.
- Example: Dissolve 25 grams of NaCl in 500 milliliters of water to make a 5% solution.
- Remember that if you are diluting a liquid substance, to obtain the required amount of water, subtract the volume of the added substance from the final volume of the solution: 500 ml - 25 ml = 475 ml of water.
Method 2 of 4: Preparation of a molecular solution
 1 Determine the molecular weight of the substance used using the formula. The formula weight (or simply molecular weight) of a compound is written in grams per mole (g / mol) on the bottle wall. If you can't find the molecular weight on the bottle, look online.
1 Determine the molecular weight of the substance used using the formula. The formula weight (or simply molecular weight) of a compound is written in grams per mole (g / mol) on the bottle wall. If you can't find the molecular weight on the bottle, look online. - The molecular weight of a substance is the mass (in grams) of one mole of that substance.
- Example: The molecular weight of sodium chloride (NaCl) is 58.44 g / mol.
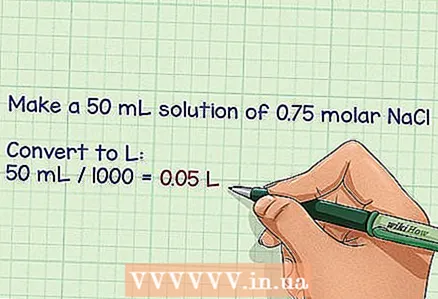 2 Determine the volume of the required solution in liters. It is very easy to prepare one liter of solution, as its molarity is expressed in moles / liter, however, it may be necessary to make more or less liters, depending on the purpose of the solution. Use the final volume to calculate the required number of grams.
2 Determine the volume of the required solution in liters. It is very easy to prepare one liter of solution, as its molarity is expressed in moles / liter, however, it may be necessary to make more or less liters, depending on the purpose of the solution. Use the final volume to calculate the required number of grams. - Example: it is necessary to prepare 50 ml of a solution with a molar fraction of NaCl of 0.75.
- To convert milliliters to liters, divide them by 1000 and get 0.05 liters.
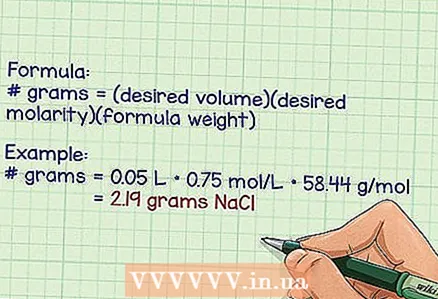 3 Calculate the number of grams required to prepare the required molecular solution. To do this, use the following formula: number of grams = (required volume) (required molarity) (molecular weight according to the formula). Remember that the required volume is expressed in liters, molarity is in moles per liter, and the molecular weight according to the formula is in grams per mole.
3 Calculate the number of grams required to prepare the required molecular solution. To do this, use the following formula: number of grams = (required volume) (required molarity) (molecular weight according to the formula). Remember that the required volume is expressed in liters, molarity is in moles per liter, and the molecular weight according to the formula is in grams per mole. - Example: if you want to prepare 50 milliliters of a solution with a molar fraction of NaCl 0.75 (molecular weight according to the formula: 58.44 g / mol), you should calculate the number of grams of NaCl.
- number of grams = 0.05 l * 0.75 mol / l * 58.44 g / mol = 2.19 grams of NaCl.
- By reducing the units of measurement, you get grams of a substance.
 4 Weigh the substance. Weigh out the required amount using a properly calibrated balance. Place a bowl on the balance and zero before weighing. Add the substance to the bowl until you reach the desired mass.
4 Weigh the substance. Weigh out the required amount using a properly calibrated balance. Place a bowl on the balance and zero before weighing. Add the substance to the bowl until you reach the desired mass. - Clean the weighing pan after use.
- Example: Weigh 2.19 grams of NaCl.
 5 Dissolve the powder in the required amount of liquid. Unless otherwise noted, most solutions use water. In this case, the same volume of liquid is taken that was used to calculate the mass of the substance. Add substance to water and stir until completely dissolved.
5 Dissolve the powder in the required amount of liquid. Unless otherwise noted, most solutions use water. In this case, the same volume of liquid is taken that was used to calculate the mass of the substance. Add substance to water and stir until completely dissolved. - Sign the container with the solution. Clearly label the solute and molarity so that you can use the solution later.
- Example: Using a beaker (volume measuring instrument), measure out 50 ml of water and dissolve 2.19 grams of NaCl in it.
- Stir the solution until the powder is completely dissolved.
Method 3 of 4: Diluting Solutions of Known Concentration
 1 Determine the concentration of each solution. When diluting solutions, you need to know the concentration of the original solution and the solution you want to obtain.This method is suitable for diluting concentrated solutions.
1 Determine the concentration of each solution. When diluting solutions, you need to know the concentration of the original solution and the solution you want to obtain.This method is suitable for diluting concentrated solutions. - Example: Prepare 75 milliliters of 1.5 M NaCl solution from a 5 M solution. The stock solution has a concentration of 5 M and it is necessary to dilute it to a concentration of 1.5 M.
 2 Determine the volume of the final solution. It is necessary to find the volume of the solution that you want to receive. You will have to calculate the amount of solution that will be required to dilute this solution to the desired concentration and volume.
2 Determine the volume of the final solution. It is necessary to find the volume of the solution that you want to receive. You will have to calculate the amount of solution that will be required to dilute this solution to the desired concentration and volume. - Example: Prepare 75 milliliters of 1.5 M NaCl solution from a 5 M starting solution. In this example, the final solution volume is 75 milliliters.
 3 Calculate the volume of solution needed to dilute the starting solution. To do this, you need the following formula: V1C1= V2C2where V1 - the volume of the required solution, C1 - its concentration, V2 - volume of the final solution, C2 - his concentration.
3 Calculate the volume of solution needed to dilute the starting solution. To do this, you need the following formula: V1C1= V2C2where V1 - the volume of the required solution, C1 - its concentration, V2 - volume of the final solution, C2 - his concentration. - To calculate the volume of the required fluid, it is necessary to rewrite the equality with respect to V1: V1 = (V2C2) / C1.
- Example: You need to prepare a 75 ml solution of NaCl with a concentration of 1.5 M from a solution with a concentration of 5 M.
- V1 = (V2C2) / C1 = (0.075 l * 1.5 M) / 5M = 0.0225 l.
- Convert liters back to milliliters by multiplying by 1000 to get 22.5 milliliters.
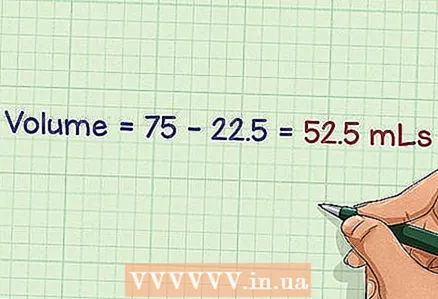 4 Subtract the volume of the original solution from the planned final volume. As a result of dilution of the solution, it is necessary to obtain a fixed final volume. Subtract the volume of the stock solution from the final volume to determine the volume of the dilution solution.
4 Subtract the volume of the original solution from the planned final volume. As a result of dilution of the solution, it is necessary to obtain a fixed final volume. Subtract the volume of the stock solution from the final volume to determine the volume of the dilution solution. - Example: The final volume is 75 milliliters and the original volume is 22.5 milliliters. Thus, we get 75 - 22.5 = 52.5 milliliters. It is this volume of liquid that will be needed to dilute the solution.
 5 Mix the calculated amount of the stock solution with the dilution liquid. Using a beaker (liquid volume measuring instrument), measure out the required amount of the stock solution and mix it with the required volume of the dilution liquid.
5 Mix the calculated amount of the stock solution with the dilution liquid. Using a beaker (liquid volume measuring instrument), measure out the required amount of the stock solution and mix it with the required volume of the dilution liquid. - Example: Measure out 22.5 milliliters of a 5M NaCl stock solution and dilute with 52.5 milliliters of water. Stir the solution.
- Write on the container with the diluted solution its concentration and composition: 1.5 M NaCl.
- Remember, if you are diluting acid with water, you should add acid to the water, but never the other way around.
Method 4 of 4: Compliance with safety precautions
 1 Use personal protective equipment. When working with aggressive chemicals and solutions, protect against their effects. Be sure to wear a lab coat, closed shoes, safety glasses and gloves.
1 Use personal protective equipment. When working with aggressive chemicals and solutions, protect against their effects. Be sure to wear a lab coat, closed shoes, safety glasses and gloves. - Use a lab coat made of non-flammable material.
- Safety goggles should have side shields that cover the eyes from the side.
 2 Work in a well-ventilated area. When the solutions are mixed, volatile gases can be released. Some substances should only be handled under a laboratory hood. If you are mixing solutions at home, open windows and turn on a fan to ensure adequate air circulation.
2 Work in a well-ventilated area. When the solutions are mixed, volatile gases can be released. Some substances should only be handled under a laboratory hood. If you are mixing solutions at home, open windows and turn on a fan to ensure adequate air circulation.  3 Add acid to water. When diluting concentrated acids, always add the acid to the water. When water and acid are mixed, an exothermic (with the release of heat) reaction occurs, which can lead to an explosion if water is added to the acid, and not vice versa.
3 Add acid to water. When diluting concentrated acids, always add the acid to the water. When water and acid are mixed, an exothermic (with the release of heat) reaction occurs, which can lead to an explosion if water is added to the acid, and not vice versa. - Remember the safety precautions every time you work with acids.
Tips
- Before getting started, familiarize yourself with the subject. Knowledge is power!
- Try using regular household products. Don't try to do anything extraordinary. If you suspect that a danger may arise, give up.
Warnings
- Don't mix bleach and ammonia.
- Wear safety equipment, goggles, a plastic apron, and neoprene gloves as needed.
What do you need
- Precise mechanical or electronic scales to determine the mass. For example, you can use a kitchen scale.
- Graduated glassware. These utensils can be found at a kitchenware store or ordered online. Measuring glass comes in a variety of shapes and sizes. Plastic dishes will work, although they will not withstand high temperatures.