Author:
Peter Berry
Date Of Creation:
11 February 2021
Update Date:
1 July 2024

Content
The periodic table of elements listed the 118 elements that have currently been discovered. There are many symbols and numbers to distinguish between elements, while the periodic table sorts elements according to their similar properties. You can read the periodic table according to the instructions below.
Steps
Part 1 of 4: Understanding structure
The periodic table starts at the top left and ends at the end of the last row, near the bottom right. The table is structured from left to right in the ascending direction of the atomic number. The atomic number is the number of protons in an atom.
- Not all rows or columns contain all the elements. Even though there may be some space in between, we continue to read the periodic table from left to right. Hydrogen, for example, has an atomic number of 1 and it is in the upper left. Helium has atomic number 2 and it is in the upper right.
- Elements 57 through element 102 are arranged in a small panel at the bottom right of the board. They are "rare earth elements".

Find a "group" of elements in each column of the periodic table. We have 18 columns.- In a group we read from top to bottom.
- The number of groups is marked above the columns; however, a few other groups are numbered below, such as the metal group.
- The numbering on the periodic table can be very different. One can use Roman numerals (IA), Arabic numerals (1A), or numbers 1 through 18.
- Hydrogen can be classified in the halogen group or the alkali metal group, or both.

Find the "period" of the element in each row of the periodic table. We have 7 cycles. In one cycle we read from left to right.- Periods are numbered 1 through 7 on the left side of the board.
- The next cycle will be larger than the previous cycle. The big concept here means that the energy level of the atom increases gradually on the periodic table.

Understand additional grouping by metals, semi-metals, and nonmetals. Color will change a lot.- The metal will be painted in the same color. However, hydrogen is often colored the same color as nonmetals and grouped with nonmetals. Metallic luster, usually solid at room temperature, is thermally conductive and conductive, ductile and malleable.
- Nonmetals are colored the same color. They are elements C-6 through Rn-86, including H-1 (Hydrogen). Nonmetals have no metallic luster, do not conduct heat or electricity, and are not ductile. They are usually gaseous at room temperature and can be solid, gaseous, or liquid.
- Semi-metallic / nonmetals are typically colored purple or green, a combination of two other colors. The diagonal line stretching from element B-5 to At-85 is the boundary line. They have some metallic properties and some nonmetallic properties.
Note that elements are sometimes also arranged in families. They are alkali metals (1A), alkaline earth metals (2A), halogen (7A), rare gases (8A) and carbon (4A).
- The prime family is numbered according to Roman, Arabic or standard numerals.
Part 2 of 4: Reading chemical symbols and element names
Read chemical symbols first. It is a combination of 1 to 2 letters used consistently in languages.
- The chemical notation is derived from the element's Latin name, or the widely known common name.
- In many cases the chemical symbol is derived from an English name, as in the case of helium, "He". However, this is not a uniform rule in chemistry. For example, iron is "Fe". For this reason, you must memorize the chemical symbol / name to quickly identify an element.
Find the common name of the element. The element's name is below the chemical symbol. It will change depending on the language of the periodic table. advertisement
Part 3 of 4: Reading atomic number
Read the periodic table according to the atomic number located in the upper or upper left center of each element cell. As mentioned, the atomic number is arranged in ascending order from the upper left corner to the lower right corner. Knowing the atomic number is the fastest way to find more information about the element.
The atomic number is the number of protons in an element's atomic nucleus.
Adding or removing protons creates another element.
Find the number of protons in the atom as well as find the number of electrons in that atom. An atom has an equal number of electrons and protons.
- Note that there is an exception to this rule. If an atom loses or accepts electrons, it becomes a charged ion.
- If there is a plus sign next to an element's chemical symbol, it is a positive charge. If it is a minus sign, it is a negative charge.
- If there is no plus or minus sign and the chemistry problem does not involve ions, you can consider that the number of protons is equal to the number of electrons.
Part 4 of 4: Atomic Weight Reading
Find atomic weight. This is the number below the element's name.
- Although the atomic weight appears to increase gradually from the upper left to the lower right, this is not always the case.
The atomic weight of most elements is indicated in decimal. Atomic weight is the total weight of the particles in the nucleus of an atom; however, this is the average mass atom of isotopes.
Use atomic weight to find the number of neutrons in the atom. Rounding the atomic weight to the nearest integer will be atomic mass. Then you subtract the number of protons from the cubic atom to get the number of neutrons.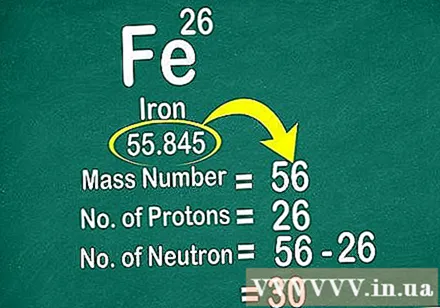
- For example, the atomic weight of iron is 55,847, so the cubic atom is 56. This atom has 26 protons. 56 (mass atom) minus 26 (proton) equals 30. That means in an iron atom there are usually 30 neutrons.
- Changing the number of neutrons in an atom results in isotopes, which are variations of atoms with heavier or lighter mass atoms.