Author:
Charles Brown
Date Of Creation:
1 February 2021
Update Date:
1 July 2024

Content
- To step
- Method 1 of 2: Accurately diluting concentrates with the dilution equation
- Method 2 of 2: Making simple, practical dilutions
- Warnings
Dilution is the process of making a concentrated solution less concentrated. There are a number of reasons why one might want to perform a dilution, ranging from the serious to the more general. For example, biochemists create new diluted solutions of their concentrated form for use in their experiments, while on the other end of the spectrum, a bartender dilutes liquor with a soda or juice to make a cocktail more delicious. The formal formula for calculating a dilution is C.1V.1= C2V.2, where C1 and C.2 represent the concentrations of the initial and final solutions, respectively, and V1 and V2 represent their volumes.
To step
Method 1 of 2: Accurately diluting concentrates with the dilution equation
 Decide what you do and don't know. Doing a chemistry dilution usually means taking a small amount of a solution of known concentration, then adding a neutral liquid (such as water) to make a new solution with a larger volume, but a lower one. concentration. This often happens in laboratories because, for efficiency reasons, the reagents are often stored in relatively high concentrations that are diluted for use in tests. In practice, you will usually know the initial concentration of the solution and the concentration and volume of your second desired solution, but not the volume of the first solution you want to use to get there.
Decide what you do and don't know. Doing a chemistry dilution usually means taking a small amount of a solution of known concentration, then adding a neutral liquid (such as water) to make a new solution with a larger volume, but a lower one. concentration. This often happens in laboratories because, for efficiency reasons, the reagents are often stored in relatively high concentrations that are diluted for use in tests. In practice, you will usually know the initial concentration of the solution and the concentration and volume of your second desired solution, but not the volume of the first solution you want to use to get there. - In other situations (especially in school assignments), you may need to find a different part of the puzzle - for example, an initial volume and concentration may have been given, instructed to determine the final concentration if you can dilute the solution to a certain volume . In the case of a dilution, it is useful to make an overview of known and unknown variables before you start.
- Let's tackle an example problem. Suppose the task is to dilute a 5 M solution with water to make 1 L of a 1 mMsolution. In this case, we know the concentration of the starting solution and the target volume and concentration that we want to achieve, but not how much of the original solution (which we are going to dilute with water) we need to get there.
- Reminder: In chemistry, M is a measure of concentration called Molarity, or the number of moles of a substance per liter.
 Use your values in the formula C.1V.1= C2V.2. In this formula, C1 the concentration of the starting solution, V.1 the volume of the starting solution, C.2 the concentration of the final solution and V.2 the volume of the final solution. Using your given values in this equation should get you the unknown value with minimal effort.
Use your values in the formula C.1V.1= C2V.2. In this formula, C1 the concentration of the starting solution, V.1 the volume of the starting solution, C.2 the concentration of the final solution and V.2 the volume of the final solution. Using your given values in this equation should get you the unknown value with minimal effort. - It may be helpful to place a question mark in front of the unit you need to determine to help you solve it.
- Let's continue with our example. We use our sample values as follows:
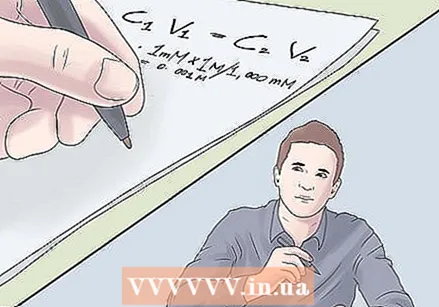
- C.1V.1= C2V.2
- (5 M) V1= (1 mM) (1 L). Our two concentrations have different units. Let's stop here and move on to the next step.
 Be aware of any differences in units. Because dilutions lead to changes in concentration (which can sometimes be quite large), it is not uncommon for two variables in your equation to have different units. While this is easily overlooked, mismatches in your equation can lead to an answer in different orders of magnitude. Before solving the problem, you must convert all values with different concentration and / or volume units.
Be aware of any differences in units. Because dilutions lead to changes in concentration (which can sometimes be quite large), it is not uncommon for two variables in your equation to have different units. While this is easily overlooked, mismatches in your equation can lead to an answer in different orders of magnitude. Before solving the problem, you must convert all values with different concentration and / or volume units. - In our example, we use different units for the concentration M (molar) and mM (millimolar). Let's convert our second measurement to M:
- 1 mM × 1 M / 1,000 mM
- = 0.001 M
- In our example, we use different units for the concentration M (molar) and mM (millimolar). Let's convert our second measurement to M:
 Solve. When all units match, solve the equation. This can almost always be done with simple algebra.
Solve. When all units match, solve the equation. This can almost always be done with simple algebra. - We continue with our example problem: (5 M) Q1= (1 mM) (1 L). Let's V1 solve with our new units.
- (5 M) V1= (0.001 M) (1 L)
- V.1= (0.001 M) (1 L) / (5 M).
- V.1=0.0002 l or 0.2 ml
- We continue with our example problem: (5 M) Q1= (1 mM) (1 L). Let's V1 solve with our new units.
 Understand how to use this answer in a practical way. Suppose you have found your missing value, but you have doubts about applying this new data to a dilution that you actually want to perform. This is understandable - the language of math and science sometimes doesn't lend itself well to the real world. If you put all four values in the equation C1V.1= C2V.2 do the dilution as follows:
Understand how to use this answer in a practical way. Suppose you have found your missing value, but you have doubts about applying this new data to a dilution that you actually want to perform. This is understandable - the language of math and science sometimes doesn't lend itself well to the real world. If you put all four values in the equation C1V.1= C2V.2 do the dilution as follows: - Measure the volume V.1 of the solution with concentration C.1. Then add enough diluent (water, etc.) to make a total volume of V.2. This new solution gives you the desired concentration (C.2).
- In our example, for example, you first measure 0.2 ml of the solution of our 5 M solution. Then add enough water to increase the volume of the solution to 1 L: 1 L - 0.0002 L = 0.9998 L or 999.8 ml. In other words, we add 999.8 ml of water to our small sample of the solution. The new, diluted solution has a concentration of 1 mM, which is what we wanted to achieve in the first place.
Method 2 of 2: Making simple, practical dilutions
 Read the information on the packaging. There are many reasons why you might want to make a dilution at home, in the kitchen, or another non-lab setting. For example, the simple act of making orange juice from concentrate is a dilution. In many cases, products that need to be diluted contain more information about the dilution on the packaging. They can even provide precise directions to follow. Here are some things to keep in mind when looking for information:
Read the information on the packaging. There are many reasons why you might want to make a dilution at home, in the kitchen, or another non-lab setting. For example, the simple act of making orange juice from concentrate is a dilution. In many cases, products that need to be diluted contain more information about the dilution on the packaging. They can even provide precise directions to follow. Here are some things to keep in mind when looking for information: - The volume of the product to be used
- The volume of the diluent to be used
- The type of diluent to use (usually water)
- Special mixing instructions
- You probably will no get information about the exact concentrations of the fluids being used. This information is unnecessary for the average consumer.
 Add the diluent to the concentrated solution. For simple household dilutions like the ones you could do in the kitchen, all you need to do before you start is really know what amount of concentrate you are using and the approximate final concentration you want to get. Dilute the concentrate with the appropriate amount of diluent (which is determined relative to the initial volume of the concentrate used. See below:
Add the diluent to the concentrated solution. For simple household dilutions like the ones you could do in the kitchen, all you need to do before you start is really know what amount of concentrate you are using and the approximate final concentration you want to get. Dilute the concentrate with the appropriate amount of diluent (which is determined relative to the initial volume of the concentrate used. See below: - For example, if we want to dilute 1 cup of concentrated orange juice to a quarter of the initial concentration, then we add 3 cups water to the concentrate. Our final blend will then have 1 cup of concentrate to 4 cups of the total liquid - a quarter of the initial concentration.
- Here's a more complex example: if we 2/3 cup of a particular concentrate to a quarter of the initial concentration, we add 2 cups of water, because 2/3 cup is a quarter of 2 & 2/3 cups of the total liquid.
- Make sure to add the substances to a vessel large enough for the desired final volume - a large bowl or similar container.
 You can ignore the volume of powders in most cases. Powder (such as certain drink mixes) added to liquids usually do not have to be regarded as a "dilution". The change in volume due to the addition of a small amount of powder to a liquid is usually small enough to be ignored. In other words, by adding small amounts of powder to a liquid, you simply add the powder to the final volume of the liquid you want to achieve.
You can ignore the volume of powders in most cases. Powder (such as certain drink mixes) added to liquids usually do not have to be regarded as a "dilution". The change in volume due to the addition of a small amount of powder to a liquid is usually small enough to be ignored. In other words, by adding small amounts of powder to a liquid, you simply add the powder to the final volume of the liquid you want to achieve.
Warnings
- Follow all safety guidelines as specified by the manufacturing company or your company. This is especially important if you want to dilute an acidic solution.
- Working with an acidic solution may require more detailed steps and safety guidelines than diluting non-acidic solutions.