Author:
Virginia Floyd
Date Of Creation:
6 August 2021
Update Date:
1 July 2024

Content
- Steps
- Method 1 of 3: The Basics
- Method 2 of 3: Determining the type of bond by electronegativity
- Method 3 of 3: Calculating Mulliken Electronegativity
- Tips
In chemistry, electronegativity is the ability of atoms to attract electrons from other atoms to them. An atom with high electronegativity attracts electrons strongly, and an atom with low electronegativity attracts electrons weakly. Electronegativity values are used to predict the behavior of various atoms in chemical compounds.
Steps
Method 1 of 3: The Basics
 1 Chemical bonds. Such bonds arise when electrons in atoms interact with each other, that is, two electrons (one from each atom) become common.
1 Chemical bonds. Such bonds arise when electrons in atoms interact with each other, that is, two electrons (one from each atom) become common. - A description of the reasons for the interaction of electrons in atoms is beyond the scope of this article.For more information on this subject, read, for example, this article.
 2 Effect of electronegativity. When two atoms attract each other's electrons, the force of attraction is not the same. An atom with a higher electronegativity attracts two electrons more strongly. An atom with a very high electronegativity attracts electrons with such a force that we are no longer talking about shared electrons.
2 Effect of electronegativity. When two atoms attract each other's electrons, the force of attraction is not the same. An atom with a higher electronegativity attracts two electrons more strongly. An atom with a very high electronegativity attracts electrons with such a force that we are no longer talking about shared electrons. - For example, in the NaCl molecule (sodium chloride, common salt), the chlorine atom has a fairly high electronegativity, and the sodium atom is rather low. So electrons are attracted to the chlorine atom and repel sodium atoms.
 3 Electronegativity table. This table includes chemical elements arranged in the same way as in the periodic table, but for each element the electronegativity of its atoms is given. Such a table can be found in chemistry textbooks, reference materials, and on the web.
3 Electronegativity table. This table includes chemical elements arranged in the same way as in the periodic table, but for each element the electronegativity of its atoms is given. Such a table can be found in chemistry textbooks, reference materials, and on the web. - You will find an excellent electronegativity table here. Note that it uses the Pauling electronegativity scale, which is the most common. However, there are other ways to calculate electronegativity, one of which will be discussed below.
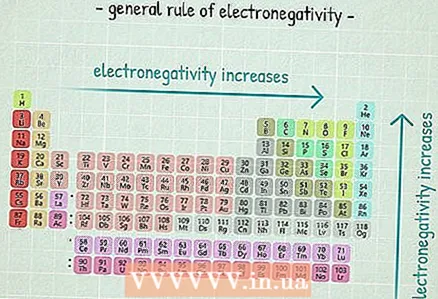 4 Electronegativity trends. If you do not have an electronegativity table at hand, you can estimate the electronegativity of an atom by the location of an element in the periodic table.
4 Electronegativity trends. If you do not have an electronegativity table at hand, you can estimate the electronegativity of an atom by the location of an element in the periodic table. - How to the right the element is located, the more the electronegativity of its atom.
- How higher the element is located, the more the electronegativity of its atom.
- Thus, the atoms of the elements located in the upper right corner of the periodic table have the highest electronegativities, and the atoms of the elements located in the lower left corner have the lowest.
- In our NaCl example, we can say that chlorine has a higher electronegativity than sodium, because chlorine is located to the right of sodium.
Method 2 of 3: Determining the type of bond by electronegativity
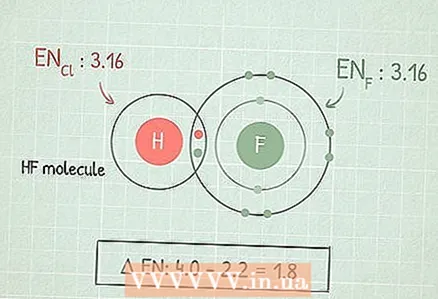 1 Calculate the difference between the electronegativities of two atoms to understand the characteristics of the bond between them. To do this, subtract the smaller electronegativity from the larger one.
1 Calculate the difference between the electronegativities of two atoms to understand the characteristics of the bond between them. To do this, subtract the smaller electronegativity from the larger one. - For example, consider the HF molecule. Subtract the electronegativity of hydrogen (2.1) from the electronegativity of fluorine (4.0): 4.0 - 2.1 = 1,9.
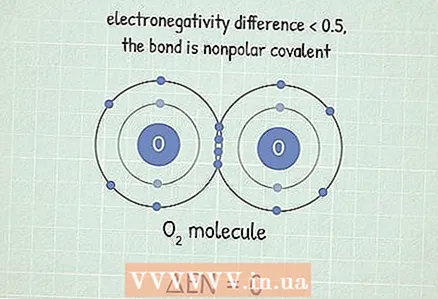 2 If the difference is less than 0.5, then the bond is covalent non-polar, in which electrons are attracted with almost the same strength. Such bonds are formed between two identical atoms. Non-polar connections are usually very difficult to break. This is because atoms share electrons, which makes their bond stable. It takes a lot of energy to destroy it.
2 If the difference is less than 0.5, then the bond is covalent non-polar, in which electrons are attracted with almost the same strength. Such bonds are formed between two identical atoms. Non-polar connections are usually very difficult to break. This is because atoms share electrons, which makes their bond stable. It takes a lot of energy to destroy it. - For example, the molecule O2 has this type of connection. Since two oxygen atoms have the same electronegativity, the difference between them is 0.
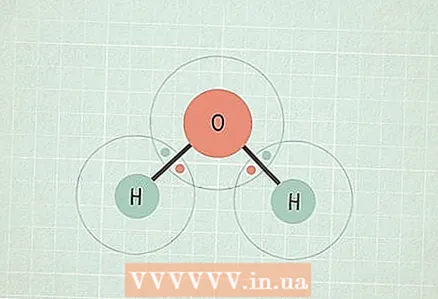
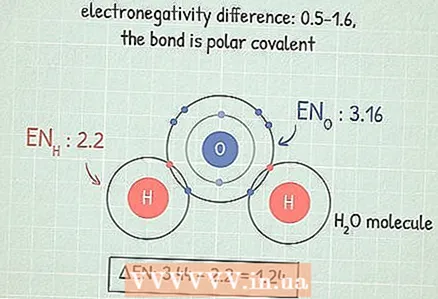 3 If the difference lies in the range of 0.5 - 1.6, then the bond is covalent polar. In this case, one of the two atoms attracts electrons more strongly and therefore acquires a partial negative charge, and the other a partial positive charge. This charge imbalance allows the molecule to participate in certain reactions.
3 If the difference lies in the range of 0.5 - 1.6, then the bond is covalent polar. In this case, one of the two atoms attracts electrons more strongly and therefore acquires a partial negative charge, and the other a partial positive charge. This charge imbalance allows the molecule to participate in certain reactions. - For example, the molecule H2O (water) has this type of bond. The O atom is more electronegative than two H atoms, so oxygen attracts electrons more strongly and acquires a partial negative charge, and hydrogen - a partial positive charge.
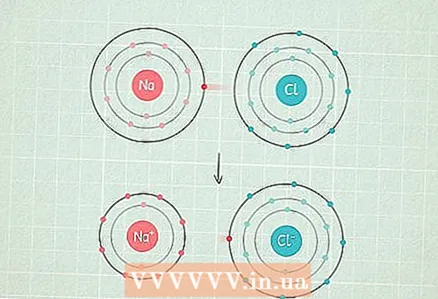
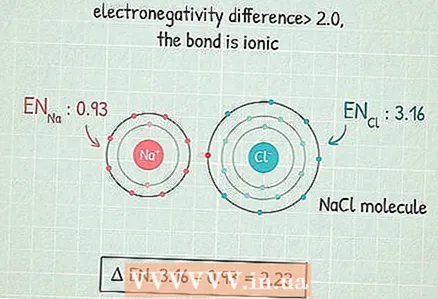 4 If the difference is greater than 2.0, then the bond is ionic. This is a bond in which the common electron pair passes predominantly to an atom with a higher electronegativity, which acquires a negative charge, and an atom with a lower electronegativity acquires a positive charge. Molecules with such bonds react well with other atoms and can even be destroyed by polar atoms.
4 If the difference is greater than 2.0, then the bond is ionic. This is a bond in which the common electron pair passes predominantly to an atom with a higher electronegativity, which acquires a negative charge, and an atom with a lower electronegativity acquires a positive charge. Molecules with such bonds react well with other atoms and can even be destroyed by polar atoms. - For example, the NaCl (sodium chloride) molecule has this type of bond.The chlorine atom is so electronegative that it attracts both electrons to itself and acquires a negative charge, and the sodium atom acquires a positive charge.
- NaCl can be destroyed by a polar molecule such as H2O (water). In a water molecule, the hydrogen side of the molecule is positive and the oxygen side is negative. If you mix salt with water, the water molecules break down the salt molecules, causing it to dissolve.
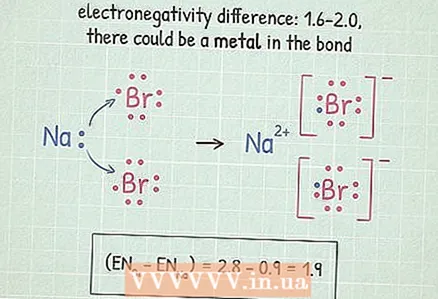 5 If the difference is between 1.6 and 2.0, check for metal. If a metal atom is present in a molecule, then the bond is ionic. If there are no metal atoms in the molecule, then the bond is polar covalent.
5 If the difference is between 1.6 and 2.0, check for metal. If a metal atom is present in a molecule, then the bond is ionic. If there are no metal atoms in the molecule, then the bond is polar covalent. - Metals are located on the left and in the center of the periodic table. In this table, metals are highlighted.
- In our HF example, the difference between electronegativities falls within this range. Since H and F are not metals, the bond polar covalent.
Method 3 of 3: Calculating Mulliken Electronegativity
 1 Find the first ionization energy of an atom. The Mulliken electronegativity scale is slightly different from the Pauling scale mentioned above. The first ionization energy is required to remove one atom from an electron.
1 Find the first ionization energy of an atom. The Mulliken electronegativity scale is slightly different from the Pauling scale mentioned above. The first ionization energy is required to remove one atom from an electron. - The meaning of such energy can be found in chemistry reference books or on the net, for example, here.
- As an example, let us find the electronegativity of lithium (Li). Its first ionization energy is 520 kJ / mol.
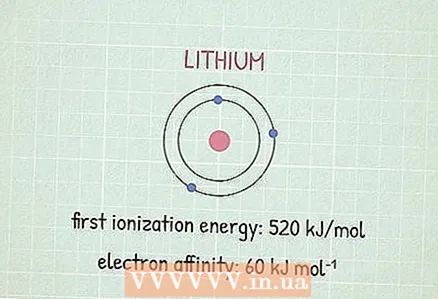 2 Find the energy of affinity for an electron. This is the energy released in the process of attaching an electron to an atom. The meaning of such energy can be found in chemistry reference books or on the net, for example, here.
2 Find the energy of affinity for an electron. This is the energy released in the process of attaching an electron to an atom. The meaning of such energy can be found in chemistry reference books or on the net, for example, here. - The electron affinity energy for lithium is 60 kJ / mol.
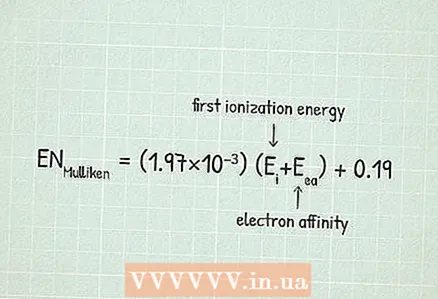
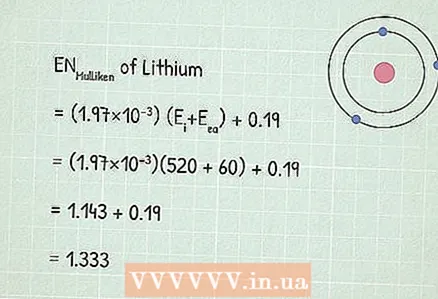 3 Use Mulliken's electronegativity equation:RUMulliken = (1.97 × 10) (Ei+ Eea) + 0,19.
3 Use Mulliken's electronegativity equation:RUMulliken = (1.97 × 10) (Ei+ Eea) + 0,19. - In our example:
- RUMulliken = (1.97 × 10) (Ei+ Eea) + 0,19
- RUMulliken = (1,97×10)(520 + 60) + 0,19
- RUMulliken = 1,143 + 0,19 = 1,333
- In our example:
Tips
- In addition to the Pauling and Mulliken scales, there are electronegativity scales according to Allred-Rochow, Sanderson, Allen. They all have their own formulas for calculating electronegativity (some of them are quite complicated).
- Electronegativity has no units of measurement.