Author:
Janice Evans
Date Of Creation:
4 July 2021
Update Date:
1 July 2024

Content
- Steps
- Method 1 of 3: Prepare a copper sulfate solution
- Method 2 of 3: Filtration of the copper sulfate solution
- Method 3 of 3: Grow copper sulfate crystals
- Warnings
- What do you need
- Similar articles
Copper sulfate is an inorganic compound that is widely used in various pesticides to combat harmful bacteria, algae, plants, snails and fungi. It is a compound of copper oxide with sulfuric acid. Copper sulfate is also often used to grow spectacular blue crystals in scientific demonstration experiments.
Attention:when conducting the experiments described below, the presence of adults is mandatory
Steps
Method 1 of 3: Prepare a copper sulfate solution
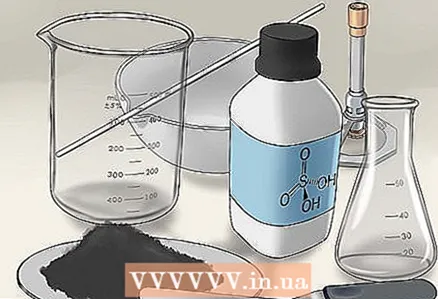 1 Collect everything you need. Place all materials and tools in one place so that you do not have to interrupt while working in search of something missing. You will need the following:
1 Collect everything you need. Place all materials and tools in one place so that you do not have to interrupt while working in search of something missing. You will need the following: - Copper oxide
- Sulfuric acid
- Protective glasses
- Glass beaker
- Conical flask
- Spatula
- Glass stirring stick
- Evaporation cup
- Bunsen burner
- Tripod
- Filter paper
- Filter funnel
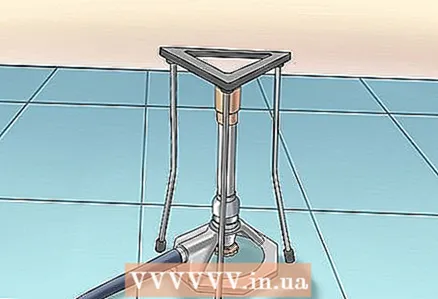 2 Prepare your workplace. Place a glass beaker on a tripod over a Bunsen burner. Wear safety glasses.
2 Prepare your workplace. Place a glass beaker on a tripod over a Bunsen burner. Wear safety glasses. 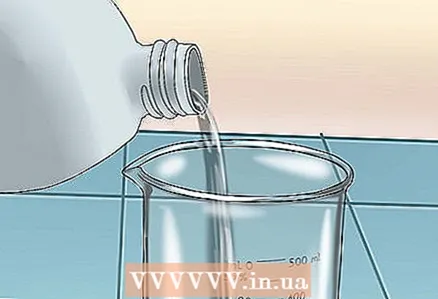 3 Pour sulfuric acid into a glass beaker. Heat it without boiling.
3 Pour sulfuric acid into a glass beaker. Heat it without boiling.  4 Add some copper oxide to the acid. Use a spatula for this so as not to burn yourself.
4 Add some copper oxide to the acid. Use a spatula for this so as not to burn yourself.  5 Stir the liquid slightly with a glass rod. Do not stir the acid too much, or it may splash on your skin. Stir for about 30 seconds each time you add more copper oxide.
5 Stir the liquid slightly with a glass rod. Do not stir the acid too much, or it may splash on your skin. Stir for about 30 seconds each time you add more copper oxide.  6 Continue heating the solution after adding all the copper oxide to it. This is necessary for a chemical reaction to proceed. It may take 1 to 2 minutes. The solution will become cloudy and a black powder will appear in it.
6 Continue heating the solution after adding all the copper oxide to it. This is necessary for a chemical reaction to proceed. It may take 1 to 2 minutes. The solution will become cloudy and a black powder will appear in it.  7 Turn off the burner. Using litmus paper, you can make sure that there is no acid left in the solution. If the acid remains, after the filtration of the solution, its vapors will appear.
7 Turn off the burner. Using litmus paper, you can make sure that there is no acid left in the solution. If the acid remains, after the filtration of the solution, its vapors will appear.  8 Set the glass of solution aside. Let the solution cool while you prepare for the filtration process.
8 Set the glass of solution aside. Let the solution cool while you prepare for the filtration process.
Method 2 of 3: Filtration of the copper sulfate solution
 1 Insert a filter funnel into the neck of the conical flask. Place filter paper in the funnel.
1 Insert a filter funnel into the neck of the conical flask. Place filter paper in the funnel. - Polyethylene filter funnels are cheaper and safer than glass ones. The diameter of the funnel should not be too large, otherwise the whole structure will be unstable.
 2 Check if the glass can be safely lifted over the funnel. If the solution is still hot, wait a little longer until you can hold the glass securely.
2 Check if the glass can be safely lifted over the funnel. If the solution is still hot, wait a little longer until you can hold the glass securely.  3 Shake the solution gently in a circular motion with the glass. Then carefully pour the liquid into the filter funnel.
3 Shake the solution gently in a circular motion with the glass. Then carefully pour the liquid into the filter funnel.  4 Wait for the solution to seep through the filter paper. As a result, there should be a clear blue liquid in the flask. If the liquid is cloudy and with a black suspension, repeat the filtration process until it clears up.
4 Wait for the solution to seep through the filter paper. As a result, there should be a clear blue liquid in the flask. If the liquid is cloudy and with a black suspension, repeat the filtration process until it clears up.
Method 3 of 3: Grow copper sulfate crystals
 1 Rinse the glass. You will need it to grow crystals. The glass must be clean so as not to contaminate the filtered solution.
1 Rinse the glass. You will need it to grow crystals. The glass must be clean so as not to contaminate the filtered solution.  2 Pour the clear blue solution into a glass. Be careful not to burn yourself when doing this, as the solution may still be hot.
2 Pour the clear blue solution into a glass. Be careful not to burn yourself when doing this, as the solution may still be hot.  3 Place the glass in a warm place for a week or longer. As the water evaporates, crystals will begin to form in it.
3 Place the glass in a warm place for a week or longer. As the water evaporates, crystals will begin to form in it. - The process of evaporation of excess water can take several weeks, depending on how warm it is in the place where the glass is stored. After the water evaporates, beautiful crystals will grow in the glass.
- Alternatively, heat the solution on a Bunsen burner and wait for half or two-thirds of the water to evaporate. Then let the solution cool down. This method is likely to result in irregularly shaped crystals.
Warnings
Please note that copper sulfate is toxic. It cannot be swallowed. Proceed with caution and always wash your hands after handling copper sulfate.
What do you need
- Copper oxide
- Sulfuric acid
- Protective glasses
- Glass beaker
- Conical flask
- Spatula
- Glass stirring stick
- Evaporation cup
- Bunsen burner
- Tripod
- Filter paper
- Filter funnel
Similar articles
- How to get copper sulfate
- How to make hot ice
- How to get distilled water
- How to understand the formula E = mc
- How to make a DNA model from ordinary materials
- How to write a hypothesis
- How to make a simple electrical circuit
- How to calculate partial pressure
- How to become a research scientist
- How to study chemistry