Author:
Alice Brown
Date Of Creation:
25 May 2021
Update Date:
1 July 2024

Content
Crystallization (or recrystallization) is the most important method for purifying organic compounds.The process of removing impurities by crystallization includes dissolving the compound in an appropriate heated solvent, cooling and saturating the solution with the compound to be purified, crystallizing it from solution, isolating it by filtration, washing with a cold solvent to remove residual impurities, and drying. This process is best done in an equipped chemistry laboratory in a well-ventilated area. Note that the procedure has a wide range of uses, including the industrial refining of sugar by crystallizing the raw product, which removes impurities from the composition.
Steps
 1 Choose a suitable solvent. Remember the aphorism like dissolves into like: Similia similibus solvuntur... For example, sugar and salt are water-soluble but not fat-soluble, and non-polar compounds such as hydrocarbons will dissolve in non-polar hydrocarbon solvents such as hexane.
1 Choose a suitable solvent. Remember the aphorism like dissolves into like: Similia similibus solvuntur... For example, sugar and salt are water-soluble but not fat-soluble, and non-polar compounds such as hydrocarbons will dissolve in non-polar hydrocarbon solvents such as hexane. - An ideal solvent has the following properties:
- It dissolves the compound when hot, but not cold.
- It either does not dissolve impurities at all (then they can be filtered from the dissolved mixture), or it dissolves them very well (in which case they will remain in solution when the desired compound is crystallized).
- It does not react with the compound being cleaned.
- Not flammable.
- It is not toxic.
- Inexpensive.
- Very volatile (therefore can be easily removed from crystals).
- It is often difficult to decide which solvent is the best; the solvent is often chosen experimentally or the most non-polar solvent available is used. See the following list of common solvents (most polar to least polar). Note that neighboring solvents on the list can mix with each other (they dissolve each other). The most commonly used solvents are shown in bold.
- Water (H2O) - non-flammable, non-toxic, cheap and dissolves many polar organic compounds; its disadvantage is its high boiling point (1000C), which makes water relatively non-volatile and makes it difficult to remove it from crystals.
- Acetic acid (CH3COOH) useful for oxidative reactions, but interacts with alcohols and amines, and therefore does not easily evaporate (boiling point at 1180C)
- Dimethyl sulfoxide (DMSO), methyl sulfoxide (CH3SOCH3) mainly used as a solvent for reactions, rarely for crystallization.
- Methanol (CH3OH) - a useful solvent that dissolves compounds that are more polar than other alcohols.
- Acetone (CH3COCH3) - good solvent; its disadvantage lies in the low boiling point (560C), which leads to small differences in the solubility of the compound at the boiling point and at room temperature.
- 2-Butanone, methyl ethyl ketone, MEK (CH3COCH2CH3) Is an excellent solvent with a boiling point of 800C.
- Ethyl acetate (CH3COOC2H5) - a very good solvent with a boiling point of 780C.
- Dichloromethane, methylene chloride (CH2Cl2) useful when mixed with naphtha, but its boiling point (350C) is too low for it to be a good solvent for crystallization.
- Diethyl ether (CH3CH2OCH2CH3) useful when mixed with naphtha, but its boiling point (400C) is too low for it to be a good solvent for crystallization.
- Methyl tert-butyl ether (CH3OC (CH3) 3) Is a cheap, good substitute for diethyl ether with a higher boiling point (520C).
- Dioxane (C4H8O2) easy to remove from crystals; weak carcinogen; forms peroxides; boiling point 1010C.
- Toluene (C6H5CH3) - an excellent solvent for the crystallization of aryl compounds, which replaced the once widely used benzene (a weak carcinogen); disadvantage - high boiling point (1110C), due to which toluene is difficult to remove from crystals.
- Pentane (C5H12)widely used for non-polar connections; often used in a mixture with another solvent.
- Hexane (C6H14) used for non-polar connections; inert; often used in mixtures; boils at 690C.
- Cyclohexane (C6H12) similar to hexane, but cheaper and boils at 810C.
- Petroleum ether is a mixture of saturated hydrocarbons, the main component of which is pentane; cheap, interchangeable with pentane; boiling point 30-600C.
- Naphtha is a mixture of saturated hydrocarbons with the properties of hexanes.
Steps for choosing a solvent
- Place a few crystals of the crude compound in a test tube and add one drop of solvent along the wall.
- If the crystals dissolve immediately at room temperature, discard the solvent as too much of the compound will remain in solution at low temperatures and try another one.
- If the crystals do not dissolve at room temperature, heat the tube in a sand bath and observe the crystals. Add another drop of solvent if they don't dissolve. If they dissolve at the boiling point of the solvent and crystallize again upon cooling to room temperature, you've found a suitable solvent. Otherwise, try another one.
- If, after trial and error, a satisfactory solvent is not found, use a mixture of two solvents. Dissolve the crystals in the best solvent (in which they almost dissolve) and add the weaker solvent to the hot solution until it becomes cloudy (saturated solute). Solvents in a pair must be miscible with each other. Some useful solvent pairs: acetic acid-water, ethanol-water, acetone-water, dioxane-water, acetone-ethanol, ethanol-diethyl ether, methanol-2-butanone, ethyl acetate-cyclohexane, acetone-ligroin, ethyl acetate-ligroin, diethyl ether-naphtha, dichloromethane-naphtha, toluene-naphtha
- An ideal solvent has the following properties:
 2 Dissolve the crude compound. To do this, place the substance in a test tube. Crush large crystals with a glass rod to speed up dissolution. Add solvent drop by drop. To remove insoluble solids, use excess solvent and filter the solution at room temperature (see step 4), then evaporate the solvent. Place a wooden stick in a test tube before heating to avoid overheating (heating the solution to a temperature above the boiling point without boiling). The air trapped in the wood will escape to form 'kernels' to ensure an even boil. Alternatively, you can use porous porcelain chips. After the solid impurities have been removed and the solvent has evaporated, add the solvent dropwise, stirring the crystals with a glass rod and heating the test tube in a steam or sand bath until the substance is completely dissolved with a minimum amount of solvent.
2 Dissolve the crude compound. To do this, place the substance in a test tube. Crush large crystals with a glass rod to speed up dissolution. Add solvent drop by drop. To remove insoluble solids, use excess solvent and filter the solution at room temperature (see step 4), then evaporate the solvent. Place a wooden stick in a test tube before heating to avoid overheating (heating the solution to a temperature above the boiling point without boiling). The air trapped in the wood will escape to form 'kernels' to ensure an even boil. Alternatively, you can use porous porcelain chips. After the solid impurities have been removed and the solvent has evaporated, add the solvent dropwise, stirring the crystals with a glass rod and heating the test tube in a steam or sand bath until the substance is completely dissolved with a minimum amount of solvent.  3 Desaturate the solution. Skip this step if the solution is colorless or has a faint yellow tint. If the solution is colored (due to high molecular weight by-products of the chemical reaction), add excess solvent and activated carbon (graphite) and boil the solution for a few minutes. Colored impurities are adsorbed on the surface of activated carbon due to its high microporosity. Remove carbon with adsorbed impurities by filtration as described in the next step.
3 Desaturate the solution. Skip this step if the solution is colorless or has a faint yellow tint. If the solution is colored (due to high molecular weight by-products of the chemical reaction), add excess solvent and activated carbon (graphite) and boil the solution for a few minutes. Colored impurities are adsorbed on the surface of activated carbon due to its high microporosity. Remove carbon with adsorbed impurities by filtration as described in the next step.  4 Removal of undissolved substances by filtration. Filtration can be done by gravity filtration, decantation, or solvent removal with a pipette. Vacuum filtration is usually not used because the hot solvent cools down and the product crystallizes on the filter.
4 Removal of undissolved substances by filtration. Filtration can be done by gravity filtration, decantation, or solvent removal with a pipette. Vacuum filtration is usually not used because the hot solvent cools down and the product crystallizes on the filter. - Gravity filtration is the best method for removing fine coal, dust, fibers, etc.Heat three Erlenmeyer flasks on a steam bath or stove: the first contains the solution to be filtered, the second contains a few milliliters of solvent and a stemless funnel, and the third contains a few milliliters of solvent that will be needed for rinsing. Place fluted filter paper (useful since you are not using vacuum) in a stemless funnel above the second flask (no tube at the end prevents the saturated solution from cooling and clogging the funnel with crystals). Bring the solution to be filtered to a boil, take the flask with a towel and pour the solution onto filter paper. Add boiling solvent from the third flask to any crystals forming on the paper, and rinse the first flask containing the solution to be filtered and pour the residue onto filter paper. Remove excess solvent from the filtered solution by boiling.
- Decantation is used for coarse solids. Simply drain (drain) the hot solvent, leaving the insoluble residue in the original container.
- Removal of solvent with a pipette: This method is used for small solution volumes and large enough solids. Place a square-nosed pipette on the bottom of the tube (round bottom) and suction up the liquid, leaving solid impurities in the tube.
 5 Crystallize the solution of interest. This step assumes that any colored and insoluble impurities have been removed in the appropriate steps described above. Remove excess solvent by boiling or blowing out with a gentle stream of air. Start with a solution saturated with a solute at the boiling point. Let it cool slowly to room temperature. Crystallization should begin. Otherwise, initiate the process by adding a seed crystal or by scratching the tube with a glass rod at the interface. When crystallization has begun, try not to touch the container so that large crystals can form. To allow for slow cooling (which allows larger crystals to form), you can insulate the container with cotton wool or paper towels. Large crystals are easier to separate from impurities. When the container is completely cooled to room temperature, chill it on ice for about five more minutes to reach the maximum amount of crystals.
5 Crystallize the solution of interest. This step assumes that any colored and insoluble impurities have been removed in the appropriate steps described above. Remove excess solvent by boiling or blowing out with a gentle stream of air. Start with a solution saturated with a solute at the boiling point. Let it cool slowly to room temperature. Crystallization should begin. Otherwise, initiate the process by adding a seed crystal or by scratching the tube with a glass rod at the interface. When crystallization has begun, try not to touch the container so that large crystals can form. To allow for slow cooling (which allows larger crystals to form), you can insulate the container with cotton wool or paper towels. Large crystals are easier to separate from impurities. When the container is completely cooled to room temperature, chill it on ice for about five more minutes to reach the maximum amount of crystals. 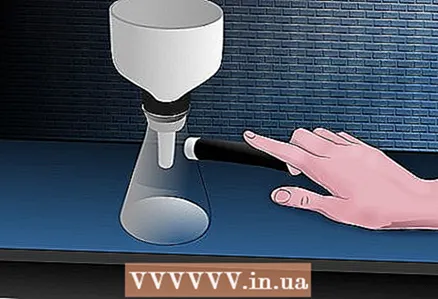 6 Collect and rinse crystals: to do this, separate the crystals from the cold solvent by filtration. This can be done with a Hirsch or Buchner funnel, or the solvent can be removed with a pipette.
6 Collect and rinse crystals: to do this, separate the crystals from the cold solvent by filtration. This can be done with a Hirsch or Buchner funnel, or the solvent can be removed with a pipette. - Filtration with a Hirsch funnel: Place a Hirsch funnel with non-corrugated filter paper in a tightly fitted vacuum tube. Place the tube on ice to keep the solvent cool. Wet filter paper with crystallization solvent. Connect the tube to the aspirator, turn it on and make sure that the filter paper is sucked into the funnel by vacuum. Pour and scrape the crystals into a funnel and turn off the aspirator once all liquid has been removed. Use a few drops of cold solvent to rinse the tube, pour the remainder into the funnel, and use the vacuum again until the liquid is removed. Wash the crystals several times with cold solvent to remove any remaining impurities. At the end of the washings, leave the aspirator on until the crystals dry.
- Filtration using a Buchner funnel: Place a piece of non-corrugated filter paper on the bottom of the Buchner funnel and wet with solvent. Place the funnel tightly into the tube using a rubber or synthetic rubber adapter to use vacuum suction. Pour and scrape the crystals into a funnel and turn off the aspirator as soon as all the liquid has been removed and the crystals remain on the paper.Rinse the crystallization tube with cold solvent, add residues to the crystals and use vacuum again until liquid is removed. Repeat and rinse crystals as many times as necessary. Leave the aspirator on until the crystals dry.
- Pipetting is used for low crystal counts. Place a square-nosed pipette on the bottom of the tube (round bottom) and suction up the liquid, leaving the washed crystals in the tube.
 7 Dry the washed product: Final drying for small amounts of crystallized product can be accomplished by drying the crystals between sheets of filter paper or leaving them to dry on a watch glass. 550px]]
7 Dry the washed product: Final drying for small amounts of crystallized product can be accomplished by drying the crystals between sheets of filter paper or leaving them to dry on a watch glass. 550px]]
Tips
- If too little solvent is used, crystallization on cooling can occur very quickly. In this case, impurities can end up inside the crystal, failing the task of purification by crystallization. On the other hand, if too much solvent is used, crystallization may not occur at all. It is best to add a lot more solvent after saturation at boiling point. Finding the right balance takes practice.
- When looking for the perfect solvent through trial and error, start with the most volatile and lowest boiling point since they are easier to remove.
- Perhaps the most important step is waiting for the hot solution to slowly cool down and crystals to form. It is imperative to be patient and let the solution cool undisturbed.
- If so much solvent has been added that small crystals form, evaporate some of the solvent while heating the solution and then re-cool.
What do you need
- Organic compound for crystallization
- Suitable solvent
- Test tubes or reaction containers
- Glass stick
- Wooden stick, or porous porcelain chips for boiling
- Activated carbon (graphite)
- Steam bath or tiles
- Erlenmeyer flasks
- Stemless funnel
- Corrugated and non-corrugated filter papers
- Pipettes
- Apparatus with Hirsch or Buchner funnel
- Watch glass