Author:
Lewis Jackson
Date Of Creation:
12 May 2021
Update Date:
1 July 2024

Content
Determining the number of neutrons in an atom is quite simple, you don't even need to do any experiments. To calculate the number of neutrons in a normal atom or isotope, you just need to have a periodic table ready and follow the instructions.
Steps
Method 1 of 2: Find the number of neutrons in a normal atom
Determine the position of the element on the periodic table. As an example, we will find the element osmium (Os) in the sixth row from the top.
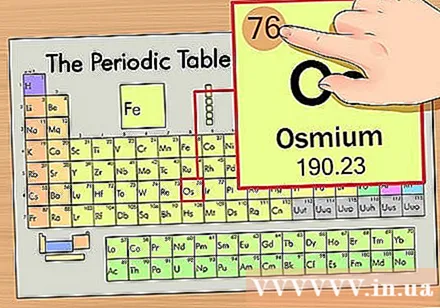
Find the atomic number of the element. This is the most conspicuous number that goes by each element and is above the prime symbol (on the board we are using there are no other numbers). Atomic number is the number of protons in a single atom of that element. Os is the number 76, which means there are 76 protons in an osmium atom.- The number of protons never changes in an element; it is essentially the defining characteristic of an element.
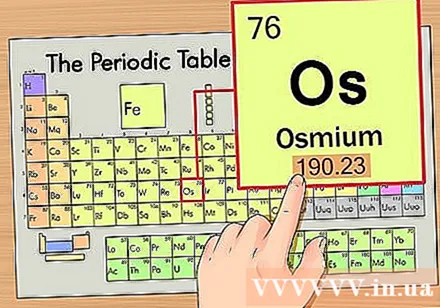
Find the atomic weight of the element. This number is usually found below the prime symbol. Note that the periodic table in this example only has atomic number and no atomic weight. Not all periodic tables. Osmium has an atomic weight of 190.23.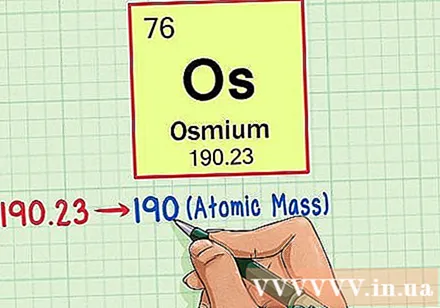
Round the atomic weight to the nearest integer to get the atomic mass. For example, 190.23 would be rounded to 190, so the atomic mass of osmium is 190.- Atomic weight is the average of isotopes of the same chemical element, which is why it is usually not an integer.
Subtract the atomic number from the atomic mass. Since most of the atomic mass is the mass of protons and neutrons, subtracting the number of protons from the atomic mass (i.e., atomic number) you will count get the number of neutrons in the atom. The number after the decimal point represents the very small mass of electrons in the atom. In this example, we have: 190 (mass atom) - 76 (number of protons) = 114 (number of neutrons).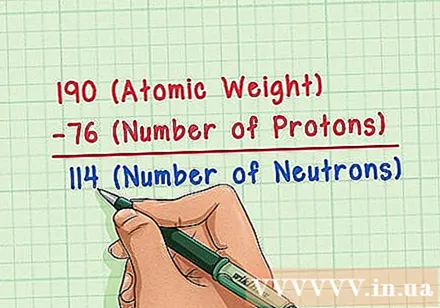
Memorize the recipe. To find the number of neutrons, we simply apply the following formula: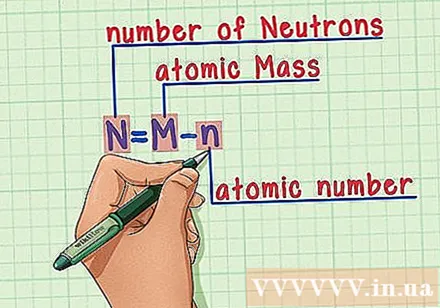
- N = M - n
- N = number of neutrons
- M = atomic mass
- n = atomic number
- N = M - n
Method 2 of 2: Find the number of neutrons in the isotope
Determine the position of the element on the periodic table. Let us take the element carbon-14 isotope as an example. Since the isotope form of carbon-14 is simply carbon (C), look for carbon on the periodic table (second row from the top).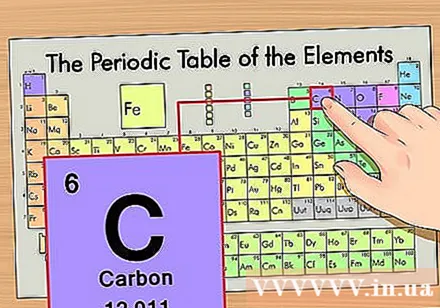
Find the atomic number of the element. This is the most conspicuous number that goes by each element and is above the prime symbol (on the board we are using there are no other numbers). Atomic number is the number of protons in a single atom of that element. C is number 6, which means there are 6 protons in a carbon atom.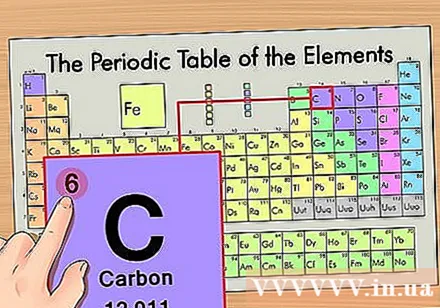
Find atomic mass. This is extremely easy with isotopes because they are named after atomic mass. For example, carbon-14 would have an atomic mass of 14. Once you have found the atomic mass of the isotope, the remaining steps to finding the number of neutrons will be the same as that of a normal atom.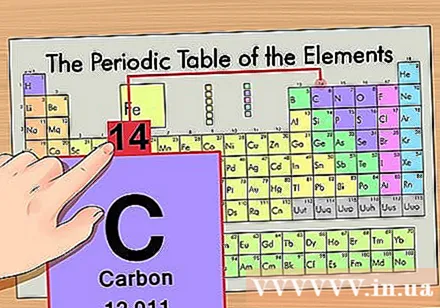
Subtract the atomic number from the atomic mass. Since most of the atomic mass is the mass of protons and neutrons, subtracting the number of protons from the atomic mass (i.e., atomic number) you will count get the number of neutrons in the atom. The number after the decimal point represents the very small mass of electrons in the atom. In this example, we have: 14 (mass atom) - 6 (number of protons) = 8 (number of neutrons).
Memorize the recipe. To find the number of neutrons, we apply the following formula: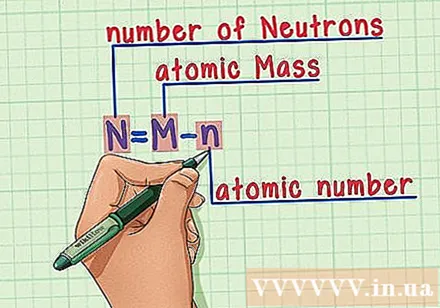
- N = M - n
- N = number of neutrons
- M = atomic mass
- n = atomic number
- N = M - n
Advice
- The mass of an element is largely the mass of protons and neutrons, while the masses of electrons and other elements are negligible (close to zero). Since the mass of the proton is approximately equal to the mass of the neutron, and the atomic number represents the number of protons, we simply subtract the number of protons from the total mass.
- If you don't remember the meaning of the numbers on the periodic table, remember that the periodic table is often built on atomic numbers (i.e. the number of protons), starting at 1 (hydrogen) and incrementing one unit from left to right, ending with 118 (ununoctium). Since the number of protons is an identifying feature of each atom, it is the simplest property on which the elements are arranged. (For example, an atom with 2 protons is always helium, just as an atom with 79 protons is always gold.)
Sources and Citations
- Interactive periodic table