Author:
Eugene Taylor
Date Of Creation:
7 August 2021
Update Date:
1 July 2024

Content
Specific heat is the amount of energy required to raise the temperature of 1 gram of a substance by 1 degree Celsius. The specific heat of a substance depends on both the molecular structure and the phase in which the substance is located. The discovery of specific heat gave impetus to the study of thermodynamics, the study of energy conversion by heat and the operation of systems. Specific heat and thermodynamics are used extensively in chemistry, nuclear research and aerodynamics, as well as in everyday life in the central heating and cooling system in your car. If you want to know how to calculate specific heat, take the following steps.
To step
Method 1 of 2: Learning the basics
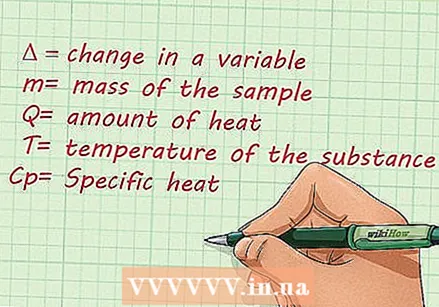 Before you learn more about the formulas to use, familiarize yourself with the terms used in calculating specific heat. Learn to recognize the different terms and what they mean. Here are the terms most commonly used when calculating the specific heat of a substance:
Before you learn more about the formulas to use, familiarize yourself with the terms used in calculating specific heat. Learn to recognize the different terms and what they mean. Here are the terms most commonly used when calculating the specific heat of a substance: - Delta, or the "Δ" symbol, represents the change of a variable.
- For example, if the first temperature (T1) is 150ºC and the second (T2) 20ºC, then ΔT, or the change in temperature, is 150ºC - 20ºC, or 130ºC.
- The mass is represented by "m".
- The amount of heat is represented by "Q". The amount of heat is represented by the "J", or Joules.
- "T" is the temperature of the substance.
- Specific heat is represented by "Cp’.
- Delta, or the "Δ" symbol, represents the change of a variable.
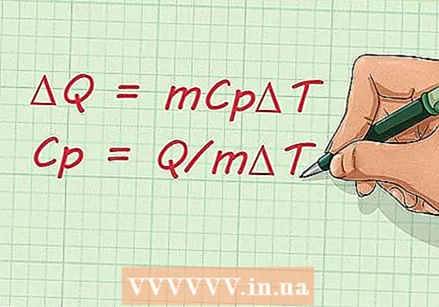 The equation of specific heat. Once you are familiar with the terms used to calculate specific heat, you should now learn the equation. The formula is: C.p = Q / mΔT.
The equation of specific heat. Once you are familiar with the terms used to calculate specific heat, you should now learn the equation. The formula is: C.p = Q / mΔT. - You can adjust this formula if you want to find the change in the amount of heat, rather than the specific heat. The equation then becomes:
- ΔQ = mCpΔT
- You can adjust this formula if you want to find the change in the amount of heat, rather than the specific heat. The equation then becomes:
Method 2 of 2: Calculating specific heat
 A closer look at the comparison. What does it take to calculate specific heat. Suppose you have the following problem: Calculate the specific heat of 350 g of an unknown substance, if you add 34,700 Joules of heat to it, and the temperature increases from 22ºC to 173ºC, without a phase change.
A closer look at the comparison. What does it take to calculate specific heat. Suppose you have the following problem: Calculate the specific heat of 350 g of an unknown substance, if you add 34,700 Joules of heat to it, and the temperature increases from 22ºC to 173ºC, without a phase change.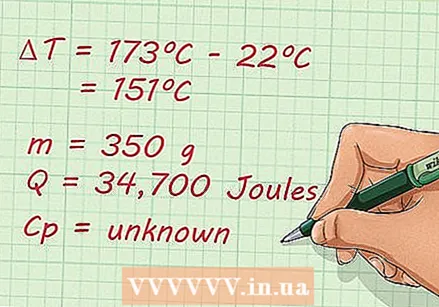 List known and unknown factors. Once the novelty of the problem is over, you can start writing down every known and unknown variable to get a better idea of what you're dealing with. This is what you should do:
List known and unknown factors. Once the novelty of the problem is over, you can start writing down every known and unknown variable to get a better idea of what you're dealing with. This is what you should do: - m = 350 g
- Q = 34,700 Joules
- ΔT = 173ºC - 22ºC = 151ºC
- C.p = unknown
 Plug the known factors into the equation. You know the value of everything except "Cpc ", so you will have to use the rest of the factors in the equation and solve for" CpThis is how it works:
Plug the known factors into the equation. You know the value of everything except "Cpc ", so you will have to use the rest of the factors in the equation and solve for" CpThis is how it works: - The original equation: C.p = Q / mΔT
- c = 34,700 J / (350 g x 151ºC)
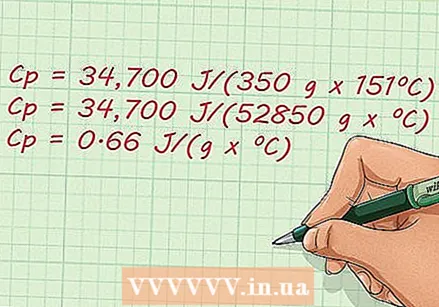 Solve the equation. Now that you've used all the known factors in the equation, the rest is simple math. The specific heat is 0.65657521286 J / (g x ºC).
Solve the equation. Now that you've used all the known factors in the equation, the rest is simple math. The specific heat is 0.65657521286 J / (g x ºC). - C.p = 34,700 J / (350 g x 151ºC)
- C.p = 34,700 J / (52850 g x ºC)
- C.p = 0.65657521286 J / (g x ºC)
Tips
- The SI (Systeme International) defines the specific heat as Joules per degree Celsius per per gram. But the number of calories per degree Fahrenheit per pound is still used in the Imperial system of units.
- Metal heats up faster than water because it has a low specific heat.
- A calorimeter can sometimes be used during a chemical reaction, when heat is being transported.
- When solving such problems, it is important to cross out the units where possible.
- The specific heat of many objects can be found in special reference books or online.
- Temperature change is greater in materials with a low specific heat, provided all other conditions remain unchanged.
- The formula for calculating the specific heat of food. C.p = 4,180 x w + 1,711 x p + 1,928 x f + 1,547 x c + 0,908 x a is the equation used to calculate the specific heat of food. "W" is the percentage of water, "p" is the percentage of protein, "f" is the percentage of fat, "c" is the percentage of carbohydrates, and "a" is the percentage of carbon. This equation takes into account the mass (x) of all the solids that make up the food. The specific heat is expressed in kJ / (kg-K).