Author:
Roger Morrison
Date Of Creation:
3 September 2021
Update Date:
1 July 2024

Content
Part of working and learning to deal with the Periodic Table is the ability to determine how many protons, neutrons and electrons go into an atom. This article tells you how!
To step
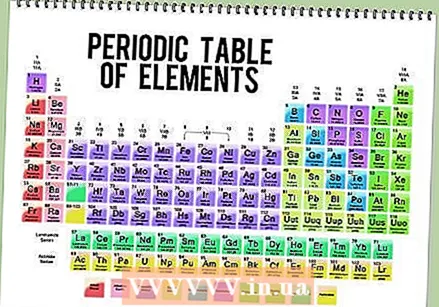 Find a picture of the periodic table. This is an overview of all chemical elements, in order (ascending) of atomic number. You can also extract other important information about an element, such as the abbreviation and atomic mass. The position in the table also indicates other significant properties of an element.
Find a picture of the periodic table. This is an overview of all chemical elements, in order (ascending) of atomic number. You can also extract other important information about an element, such as the abbreviation and atomic mass. The position in the table also indicates other significant properties of an element. 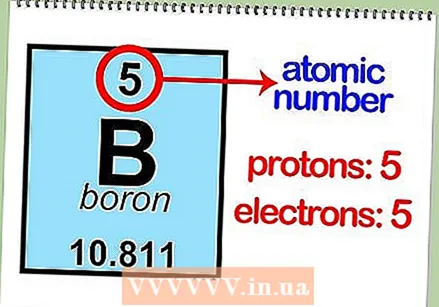 Read the atomic number of an element. With this you can determine the number of protons and electrons. The atomic number is above the element's symbol in the box. For example, boron (B) has an atomic number of 5, which means that it has 5 protons and 5 electrons.
Read the atomic number of an element. With this you can determine the number of protons and electrons. The atomic number is above the element's symbol in the box. For example, boron (B) has an atomic number of 5, which means that it has 5 protons and 5 electrons. 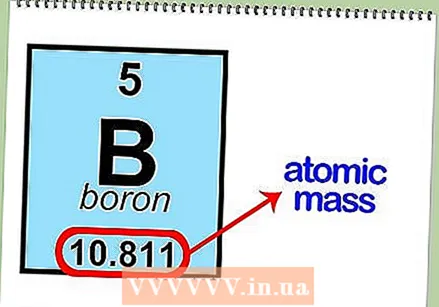 Determine the atomic mass of the element. You can usually find this number under the symbol of the atom. The atomic mass of boron is 10,811.
Determine the atomic mass of the element. You can usually find this number under the symbol of the atom. The atomic mass of boron is 10,811. 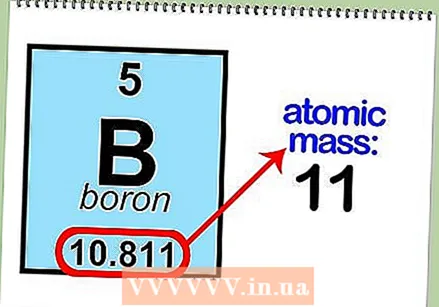 Round atomic mass to the nearest whole number to find atomic mass. For example, the rounded atomic mass of boron is 11.
Round atomic mass to the nearest whole number to find atomic mass. For example, the rounded atomic mass of boron is 11. 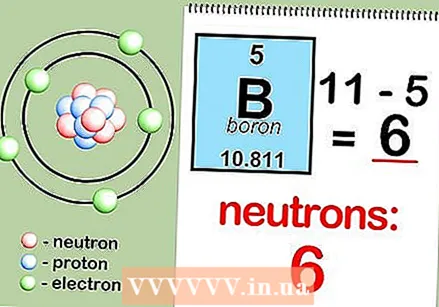 Subtract the atomic number from the atomic mass. Since most of the atomic mass is determined by the protons and neutrons, by subtracting the number of protons (the atomic number) from the atomic mass, you can calculate the number of neutrons in the atom. For example: 11 (atomic mass) - 5 (number of protons) = 6 (number of neutrons).
Subtract the atomic number from the atomic mass. Since most of the atomic mass is determined by the protons and neutrons, by subtracting the number of protons (the atomic number) from the atomic mass, you can calculate the number of neutrons in the atom. For example: 11 (atomic mass) - 5 (number of protons) = 6 (number of neutrons). - Remember the formula. To find the number of neutrons in an atom in the future, just remember the formula number of neutrons = atomic mass - atomic number.