Author:
Marcus Baldwin
Date Of Creation:
20 June 2021
Update Date:
22 June 2024

Content
- Steps
- Method 1 of 5: Good Study Habits
- Method 2 of 5: Understanding the atomistic structure
- Method 3 of 5: Calculating Chemical Reactions
- Method 4 of 5: Calculations
- Method 5 of 5: The Language of Chemistry
- Tips
To pass the General Chemistry exam, it is important to know the basics of the subject, be able to count, use a calculator for more complex problems, and be ready to learn something new. Chemistry studies substances and their properties. Everything around us is about chemistry, even the simplest things we take for granted, like the water we drink and the air we breathe. Get ready for discoveries about everything that surrounds you. Getting to know chemistry will be fun.
Steps
Method 1 of 5: Good Study Habits
 1 Meet your teacher or teacher. To pass the exam successfully, you should get to know your instructor and tell him what is difficult for you.
1 Meet your teacher or teacher. To pass the exam successfully, you should get to know your instructor and tell him what is difficult for you. - Many teachers can be approached outside of class if students need help. In addition, they usually have methodical publications.
 2 Get a group together to practice. Do not be ashamed that chemistry is hard for you. This subject is difficult for almost everyone.
2 Get a group together to practice. Do not be ashamed that chemistry is hard for you. This subject is difficult for almost everyone. - Working in a group, people who can quickly understand a topic will explain it to others. Divide and Conquer.
 3 Read the relevant paragraphs in the tutorial. A chemistry textbook is not the most exciting reading, but you should carefully read the material and highlight the text that you do not understand. Make a list of questions and concepts that are difficult for you to understand.
3 Read the relevant paragraphs in the tutorial. A chemistry textbook is not the most exciting reading, but you should carefully read the material and highlight the text that you do not understand. Make a list of questions and concepts that are difficult for you to understand. - Come back to these parts later with a fresh head. If you still find it difficult, discuss the topic in a group or ask your teacher for help.
 4 Answer the questions after the paragraph. Even if there is a lot of material, you may have memorized more than you think. Try to answer the questions at the end of the chapter.
4 Answer the questions after the paragraph. Even if there is a lot of material, you may have memorized more than you think. Try to answer the questions at the end of the chapter. - Sometimes textbooks have explanatory material at the end that describes the correct solution. This will help you understand where you went wrong in reasoning.
 5 Examine charts, images, and tables. The textbooks use visual means of conveying information.
5 Examine charts, images, and tables. The textbooks use visual means of conveying information. - Look at pictures and diagrams. This will allow you to better understand some of the concepts.
 6 Ask your instructor for permission to record the lecture on a tape recorder. It is difficult to write down information and still look at the blackboard, especially when it comes to such a complex subject as chemistry.
6 Ask your instructor for permission to record the lecture on a tape recorder. It is difficult to write down information and still look at the blackboard, especially when it comes to such a complex subject as chemistry.  7 Check out the previous exam questions. Sometimes students are given questions that were encountered in exams in previous years so that they can better prepare.
7 Check out the previous exam questions. Sometimes students are given questions that were encountered in exams in previous years so that they can better prepare. - Don't memorize the answers. Chemistry is a subject where, in order to answer a question, it is important to understand what it is about, and not just repeat a memorized text.
 8 Take advantage of online learning resources. Visit all the sites that your instructor recommends.
8 Take advantage of online learning resources. Visit all the sites that your instructor recommends.
Method 2 of 5: Understanding the atomistic structure
 1 Start with the simplest building. To become an exam, you will need to know what everything is made of, which is substance and has mass.
1 Start with the simplest building. To become an exam, you will need to know what everything is made of, which is substance and has mass. - It all starts with understanding the structure of the atom. Everything else will be added from above. It is important to study all the information about the atom very carefully.
 2 Check out the concept of the atom. An atom is the smallest "brick" of everything that has mass, including substances that we cannot always see (for example, gases). But even an atom contains tiny particles that form its structure ..
2 Check out the concept of the atom. An atom is the smallest "brick" of everything that has mass, including substances that we cannot always see (for example, gases). But even an atom contains tiny particles that form its structure .. - An atom consists of three parts - neutrons, protons and electrons. The center of an atom is called the nucleus. The nucleus is composed of neutrons and protons. Electrons are particles that revolve around the outer shell of an atom like planets around the sun.
- The atom is very small. Imagine the largest stadium you know. If the stadium is an atom, then the nucleus of this atom is the size of a pea.
 3 Find out what the atomic structure of an element is. An element is a substance in nature that cannot be broken down into smaller substances. Elements are made up of atoms.
3 Find out what the atomic structure of an element is. An element is a substance in nature that cannot be broken down into smaller substances. Elements are made up of atoms. - The atoms in the element do not change. This means that each element has a certain unique number of neutrons and protons in its atomic structure.
 4 Find out how the kernel works. The neutrons in the nucleus have a neutral charge. Protons have a positive charge. The atomic number of an element is equal to the number of protons in the nucleus ..
4 Find out how the kernel works. The neutrons in the nucleus have a neutral charge. Protons have a positive charge. The atomic number of an element is equal to the number of protons in the nucleus .. - There is no need to count the number of protons in the nucleus. This number is indicated in the periodic table of chemical elements for each element.
 5 Count the number of neutrons in the nucleus. You can use a number from the periodic table. The atomic number of an element is the same as the number of protons in the nucleus.
5 Count the number of neutrons in the nucleus. You can use a number from the periodic table. The atomic number of an element is the same as the number of protons in the nucleus. - The atomic mass is indicated at the bottom of the square of each element under its name.
- Remember that there are only protons and neutrons in the nucleus of an atom. In the periodic table, the number of protons and the value of the atomic mass are indicated.
- Now everything will be easy to calculate. Subtract the number of protons from the atomic mass and you get the number of neutrons in the nucleus of each atom of the element.
 6 Count the number of electrons. Remember that particles with opposite charges attract. Electrons are positively charged and revolve around the atom. The number of negatively charged electrons that are attracted to the nucleus depends on the number of positively charged protons in the nucleus.
6 Count the number of electrons. Remember that particles with opposite charges attract. Electrons are positively charged and revolve around the atom. The number of negatively charged electrons that are attracted to the nucleus depends on the number of positively charged protons in the nucleus. - Since the atom itself has a neutral charge, the number of particles with a negative charge must equal the number of particles with a positive charge. For this reason, the number of electrons is equal to the number of protons.
 7 Refer to the periodic table. If the properties of elements are difficult for you, study all the available information about the periodic table.
7 Refer to the periodic table. If the properties of elements are difficult for you, study all the available information about the periodic table. - Understanding the periodic table is essential to successfully passing the exam.
- The periodic table consists of elements only. Each element has an alphabetic symbol, this symbol always denotes that element. For example, Na is always sodium. The full name of the element is placed under the letter symbol.
- The number above the letter symbol is an atomic number. It is equal to the number of protons in the nucleus.
- The number under the letter symbol is the atomic mass. Remember that atomic mass is the sum of the protons and neutrons in the nucleus.
 8 Learn to read the spreadsheet. There is a lot of information in the table, from the colors of the columns to the arrangement of elements from left to right and top to bottom.
8 Learn to read the spreadsheet. There is a lot of information in the table, from the colors of the columns to the arrangement of elements from left to right and top to bottom.
Method 3 of 5: Calculating Chemical Reactions
 1 Write an equation. In chemistry class, you will be taught to determine what happens to the elements when they are combined. On paper, this is called solving an equation.
1 Write an equation. In chemistry class, you will be taught to determine what happens to the elements when they are combined. On paper, this is called solving an equation. - The chemical equation consists of substances on the left side, an arrow and a reaction product. Substances on one side of the equation must balance substances on the other side.
- For example, substance 1 + substance 2 → product 1 + product 2.
- Take tin (Sn) in oxidized form (SnO2) and combine with hydrogen in the form of gas (H2). SnO2 + H2 → Sn + H2O.
- This equation must be balanced, since the amount of reagent substances must be equal to the amount of products obtained. There are more oxygen atoms on the left side than on the right.
- Substitute two hydrogen units on the left and two water molecules on the right. In the final version, the balanced equation looks like this: SnO2 + 2 H2 → Sn + 2 H2O.
 2 Think about equations in a new way. If you find it difficult to balance the equations, imagine this is a recipe but needs to be adjusted on both sides.
2 Think about equations in a new way. If you find it difficult to balance the equations, imagine this is a recipe but needs to be adjusted on both sides. - In the task, you are given the ingredients on the left side, but it does not say how much you need to take. The equation also says what will happen, but does not say how much. You need to find out.
- Using the previous equation as an example, SnO2 + H2 → Sn + H2O, let's think about why this formula won't work. The amount of Sn is equal on both sides, as is the amount of H2, but on the left there are two parts of oxygen, and on the right there is only one.
- It is necessary to change the right side of the equation so that the resulting product contains two parts of H2O. A two in front of H2O means that all quantities will be doubled. The oxygen is now balanced, but a 2 means there is now more hydrogen on the right than on the left. Go back to the left side and double the hydrogen by placing a two in front of it.
- Everything is now in balance. Input quantities are equal to output quantities.
 3 Add more detail to the equation. In chemistry classes, you will get acquainted with the symbols that indicate the physical state of the elements: t - solid, g - gas, w - liquid.
3 Add more detail to the equation. In chemistry classes, you will get acquainted with the symbols that indicate the physical state of the elements: t - solid, g - gas, w - liquid.  4 Learn to identify the changes that occur during a chemical reaction. Chemical reactions begin with basic elements or compounds that react. As a result of the connection, a reaction product or several products is obtained.
4 Learn to identify the changes that occur during a chemical reaction. Chemical reactions begin with basic elements or compounds that react. As a result of the connection, a reaction product or several products is obtained. - To pass the exam, you need to know how to solve equations that contain reactants or compound products, or both.
 5 Learn different types of reactions. Chemical reactions can occur under the influence of various factors, and not only when the elements are combined.
5 Learn different types of reactions. Chemical reactions can occur under the influence of various factors, and not only when the elements are combined. - The most common types of reactions are synthesis, analysis, substitution, double decomposition, reaction between acids and bases, oxidation-reduction, combustion, isomerization, hydrolysis.
- In the classroom, different reactions can be studied - it all depends on the objectives of the course.At the university, the degree of in-depth in the material will differ from the school curriculum.
 6 Use all available resources. You will need to understand the difference between basic reactions. Use every possible material to understand this difference. Don't be afraid to ask questions.
6 Use all available resources. You will need to understand the difference between basic reactions. Use every possible material to understand this difference. Don't be afraid to ask questions. - It is not so easy to understand what changes during chemical reactions. This will be one of the most challenging tasks in your chemistry class.
 7 Think about the reactions in terms of logic. Try not to get confused by the terminology and make things even more complicated. All reactions are aimed at transforming something into something else.
7 Think about the reactions in terms of logic. Try not to get confused by the terminology and make things even more complicated. All reactions are aimed at transforming something into something else. - For example, you already know what happens if you combine two hydrogen atoms and one oxygen atom - water. Therefore, if you pour water into a saucepan and put it on fire, something will change. You have carried out a chemical reaction. If you put water in the refrigerator, a reaction will happen. You changed something that involved a reactant, which is water.
- Go through each type of reaction until you understand everything. Concentrate on the source of energy that triggers the reaction and the major changes that result from the reaction.
- If you find it difficult to understand this, make a list of incomprehensible nuances and show it to your teacher, fellow students, or anyone who is well versed in chemistry.
Method 4 of 5: Calculations
 1 Know the sequence of basic calculations. In chemistry, sometimes very accurate calculations are needed, but often a basic knowledge of mathematics is enough. It is important to understand in what sequence the calculations are carried out.
1 Know the sequence of basic calculations. In chemistry, sometimes very accurate calculations are needed, but often a basic knowledge of mathematics is enough. It is important to understand in what sequence the calculations are carried out. - First, calculations are done in brackets, then calculations in powers, then multiplication or division, and at the end - addition or subtraction.
- In example 3 + 2 x 6 = ___, the correct answer is 15.
 2 Don't be afraid to round very long numbers. In chemistry, they often round off, because often the answer to an equation is a number with a large number of digits. If there are instructions for rounding in the problem statement, take them into account.
2 Don't be afraid to round very long numbers. In chemistry, they often round off, because often the answer to an equation is a number with a large number of digits. If there are instructions for rounding in the problem statement, take them into account. - Know how to round. If the next digit is 4 or less, it should be rounded down, if 5 or more than 5, it should be rounded up. For example, here is the number 6.66666666666666. The task says to round the answer to the second digit after the dot. The answer is 6.67.
 3 Understand what absolute value is. In chemistry, some numbers have an absolute, not a mathematical, meaning. An absolute value is all values up to a number from zero.
3 Understand what absolute value is. In chemistry, some numbers have an absolute, not a mathematical, meaning. An absolute value is all values up to a number from zero. - In other words, you no longer have negative and positive values, only the distance to zero. For example, the absolute value of -20 is 20.
 4 Know all common units of measurement. Here are some examples.
4 Know all common units of measurement. Here are some examples. - The amount of a substance is measured in moles (mol).
- Temperature is measured in degrees Fahrenheit (° F), Kelvin (° K), or Celsius (° C).
- Mass is measured in grams (g), kilograms (kg) or milligrams (mg).
- The volume of the liquid is measured in liters (l) or milliliters (ml).
 5 Practice translating values from one measurement system to another. On the exam, you will have to deal with such translations. You may need to convert the temperature from one system to another, pounds to kilograms, ounces to liters.
5 Practice translating values from one measurement system to another. On the exam, you will have to deal with such translations. You may need to convert the temperature from one system to another, pounds to kilograms, ounces to liters. - You may be asked to give your answer in units other than the units in the problem statement. For example, in the text of the problem, the temperature will be indicated in degrees Celsius, and the answer will be needed in degrees Kelvin.
- Usually the temperature of chemical reactions is measured in degrees Kelvin. Practice converting Celsius to Fahrenheit or Kelvin.
 6 Do not hurry. Read the text of the problem thoughtfully and learn how to convert units of measurement.
6 Do not hurry. Read the text of the problem thoughtfully and learn how to convert units of measurement.  7 Know how to calculate concentration. Hone your knowledge of basic math by calculating percentages, ratios and proportions.
7 Know how to calculate concentration. Hone your knowledge of basic math by calculating percentages, ratios and proportions.  8 Practice with the nutritional data on the packaging. To pass chemistry, you need to be able to calculate ratios, proportions, and percentages in different sequences.If this is difficult for you, start training in familiar units of measurement (for example, on food packaging).
8 Practice with the nutritional data on the packaging. To pass chemistry, you need to be able to calculate ratios, proportions, and percentages in different sequences.If this is difficult for you, start training in familiar units of measurement (for example, on food packaging). - Take the nutritional data pack. You will see the calculation of calories per serving, recommended food serving per day as a percentage, total fat, calories from fat percentage, total carbohydrates, and a breakdown by carbohydrate type. Learn to calculate different ratios based on these values.
- For example, calculate the amount of monounsaturated fat in total fat. Convert to percentage. Calculate the number of calories in a pack by knowing the number of servings and the calorie content of each serving. Calculate how much sodium is in half of the package.
- This will help you easily translate chemical values from one system to another, for example, moles per liter, grams per mole, and so on.
 9 Learn to use Avogadro's number. This number reflects the number of molecules, atoms or particles in one mole. Avogadro's constant is 6.022x1023.
9 Learn to use Avogadro's number. This number reflects the number of molecules, atoms or particles in one mole. Avogadro's constant is 6.022x1023. - For example, how many atoms are there in 0.450 moles of Fe? Answer: 0.450 x 6.022x1023.
 10 Think about carrots. If you find it difficult to figure out how to use Avogadro's number, try counting carrots rather than atoms, molecules, or particles. How many carrots are there in a dozen? We know that a dozen is 12, which means there are 12 carrots in one dozen.
10 Think about carrots. If you find it difficult to figure out how to use Avogadro's number, try counting carrots rather than atoms, molecules, or particles. How many carrots are there in a dozen? We know that a dozen is 12, which means there are 12 carrots in one dozen. - Now let's answer the question, how many carrots are there in a mole. Instead of multiplying by 12, we multiply by Avogadro's number. There are 6.022 x 1023 carrots in a mole.
- Avogadro's number is used to convert any value of atoms, molecules, particles or carrots to moles.
- If you know the number of moles of a substance, then the value of the number of molecules, atoms or particles will be equal to this number multiplied by Avogadro's number.
- Understanding how particles are converted to moles is an important factor in the exam. Mole conversions are part of the ratio and proportion calculations. It means the amount of something in moles as part of something else.
 11 Understand molarity. Think about the number of moles of a substance in a liquid. It is very important to understand this example because it is about molarity, that is, the proportion of a substance expressed in moles per liter.
11 Understand molarity. Think about the number of moles of a substance in a liquid. It is very important to understand this example because it is about molarity, that is, the proportion of a substance expressed in moles per liter. - Molarity, or molar concentration, is a term that expresses the amount of a substance in a liquid, that is, the amount of a solute in a solution. To obtain molarity, you need to divide the moles of the solute by the liters of solution. Molarity is expressed in moles per liter.
- Calculate the density. Density is often used in chemistry. Density is the mass of a chemical per unit volume. Typically, density is expressed in grams per milliliter or grams per cubic centimeter - it's the same thing.
 12 Reduce equations to an empirical formula. This means that the answer will be correct only if you bring all the values to their simplest form.
12 Reduce equations to an empirical formula. This means that the answer will be correct only if you bring all the values to their simplest form. - This does not apply to molecular formulas, since they indicate the exact proportions of the chemical elements that make up the molecule.
 13 Know what is included in the molecular formula. The molecular formula does not need to be brought to the simplest, or empirical, form, since it says what exactly the molecule is made of.
13 Know what is included in the molecular formula. The molecular formula does not need to be brought to the simplest, or empirical, form, since it says what exactly the molecule is made of. - The molecular formula is written using the abbreviations of the elements and the number of atoms of each element in the molecule.
- For example, the molecular formula of water is H2O. This means that each water molecule contains two hydrogen atoms and one oxygen atom. The molecular formula of acetaminophen is C8H9NO2. Every chemical compound has a molecular formula.
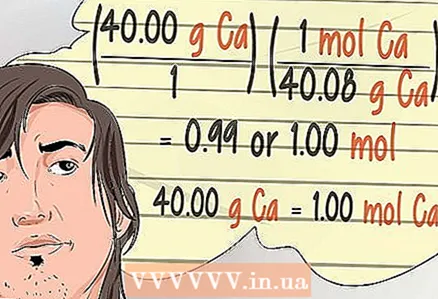 14 Remember that mathematics in chemistry is called stoichiometry. You will come across this term. This is a description of how chemistry is expressed in mathematical formulas. In chemical mathematics, or stoichiometry, the amounts of elements and chemical compounds are often expressed in moles, percentages in moles, moles per liter, or moles per kilogram.
14 Remember that mathematics in chemistry is called stoichiometry. You will come across this term. This is a description of how chemistry is expressed in mathematical formulas. In chemical mathematics, or stoichiometry, the amounts of elements and chemical compounds are often expressed in moles, percentages in moles, moles per liter, or moles per kilogram. - You will need to convert grams to moles.The atomic mass of a unit of an element in grams is equal to one mole of this substance. For example, the atomic mass of calcium is 40 atomic mass units. Thus, 40 grams of calcium equals one mole of calcium.
 15 Ask for additional assignments. If equations and conversions are difficult for you, talk to your teacher. Ask for more tasks so that you can work on them yourself until you understand the essence of all the phenomena.
15 Ask for additional assignments. If equations and conversions are difficult for you, talk to your teacher. Ask for more tasks so that you can work on them yourself until you understand the essence of all the phenomena.
Method 5 of 5: The Language of Chemistry
 1 Learn to understand Lewis charts. Lewis charts are sometimes called scatter charts. These are simple diagrams, in which dots represent free and bound electrons in the outer shell of an atom.
1 Learn to understand Lewis charts. Lewis charts are sometimes called scatter charts. These are simple diagrams, in which dots represent free and bound electrons in the outer shell of an atom. - Such a system allows you to draw simple diagrams that would reflect the bonds between elements in an atom or molecule, for example, covalent.
 2 Learn what the octet rule is. When constructing Lewis diagrams, the octet rule is used, which states that an atom becomes stable when it has access to eight electrons in its outer shell. Hydrogen is an exception - it is considered stable when there are two electrons in the outer shell.
2 Learn what the octet rule is. When constructing Lewis diagrams, the octet rule is used, which states that an atom becomes stable when it has access to eight electrons in its outer shell. Hydrogen is an exception - it is considered stable when there are two electrons in the outer shell.  3 Draw a Lewis diagram. The letter symbol of the element is surrounded by dots and is a Lewis diagram. Imagine the diagram is a movie frame. Electrons do not revolve around the outer shell of the elements - they are reflected in a certain period of time.
3 Draw a Lewis diagram. The letter symbol of the element is surrounded by dots and is a Lewis diagram. Imagine the diagram is a movie frame. Electrons do not revolve around the outer shell of the elements - they are reflected in a certain period of time. - The diagram depicts the stationary mass of electrons, the places of their connection with another element and information about the bond (for example, whether bonds are doubled and are they divided between several electrons).
- Think of the octet rule and imagine an element symbol - for example, C (carbon). Draw two dots each in the east, west, north, and south of the symbol. Now draw an H (hydrogen atom) symbol on each side of each of the dots. The diagram shows that each carbon atom is surrounded by four hydrogen atoms. Their electrons are covalently bonded, that is, for carbon and hydrogen atoms, one of the electrons is bonded to an electron of the second element.
- The molecular formula of such a compound is CH4. It is methane gas.
 4 Understand how electrons bind elements. Lewis diagrams represent chemical bonds in a simple form.
4 Understand how electrons bind elements. Lewis diagrams represent chemical bonds in a simple form. - Discuss this topic with your teacher and classmates if you do not understand how the elements are connected and what the Lewis diagrams represent.
 5 Find out what the connections are called. Chemistry has its own rules of terminology. The types of reactions, the loss or gain of electrons in the outer shell, and the stability or instability of elements are part of the terminology of chemistry.
5 Find out what the connections are called. Chemistry has its own rules of terminology. The types of reactions, the loss or gain of electrons in the outer shell, and the stability or instability of elements are part of the terminology of chemistry.  6 Take this seriously. Many chemistry courses have separate chapters for this. Often, not knowing the terminology means failing the exam.
6 Take this seriously. Many chemistry courses have separate chapters for this. Often, not knowing the terminology means failing the exam. - If possible, study the terminology before class. You can buy specialty literature at a regular bookstore or on the internet.
 7 Know what the numbers above and below the line mean. This is a very important part of learning chemistry.
7 Know what the numbers above and below the line mean. This is a very important part of learning chemistry. - The numbers above the line can be seen in the periodic table of elements. They represent the total charge of an element or chemical compound. Examine the periodic table and elements in vertical rows that have the same index numbers.
- The numbers at the bottom of the line are used to describe the amount of each element that goes into the compound. As mentioned earlier, the 2 in the H2O formula indicates that there are two hydrogen atoms in the water molecule.
 8 Understand how atoms react with each other. In terminology, there are special rules that should be followed when naming products of certain types of reactions.
8 Understand how atoms react with each other. In terminology, there are special rules that should be followed when naming products of certain types of reactions. - One of the reactions is oxidation-reduction. During the reaction, either the acquisition or the loss of electrons occurs.
- Electrons are lost during oxidation and acquired during reduction.
 9 Remember that the numbers at the bottom of the line may indicate the compound's stable charge formula. Scientists use numbers like this to describe the final molecular formula of a compound, which also denotes a stable compound with a neutral charge.
9 Remember that the numbers at the bottom of the line may indicate the compound's stable charge formula. Scientists use numbers like this to describe the final molecular formula of a compound, which also denotes a stable compound with a neutral charge. - To obtain a neutral charge, a positively charged ion, called a cation, must be balanced with an equal charge from a negative ion, an anion. These charges are written at the bottom of the line.
- For example, in the magnesium ion there is +2 the charge of the cation, and in the nitrogen ion there is -3 the charge of the anion. +2 and -3 are indicated at the bottom of the line. To obtain a neutral charge, for every 2 units of nitrogen, you need to use 3 atoms of magnesium.
- In the formula, this is written as follows: Mg3N2
 10 Learn to recognize anions and cations by their position in the periodic table of elements. The elements in the table that are in the first column are alkali metals and have +1 cation charge. For example, Na + and Li +.
10 Learn to recognize anions and cations by their position in the periodic table of elements. The elements in the table that are in the first column are alkali metals and have +1 cation charge. For example, Na + and Li +. - The alkaline earth metals in the second column have a 2+ cation charge, such as Mg2 + and Ba2 +.
- The elements in the seventh column are called halogens and have a charge of -1 anions such as Cl- and I-.
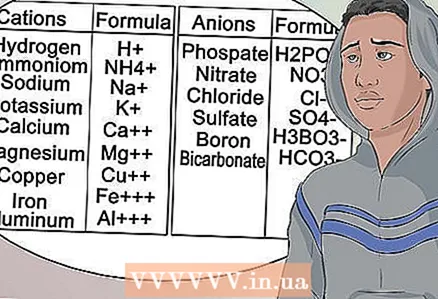 11 Learn to recognize common anions and cations. To pass the exam, learn all of the terminology associated with item groups. These numbers at the bottom of the line do not change.
11 Learn to recognize common anions and cations. To pass the exam, learn all of the terminology associated with item groups. These numbers at the bottom of the line do not change. - In other words, magnesium is always Mg with a +2 cation charge.
 12 Try not to get confused by the information. Information about different types of chemical reactions, about the exchange of electrons, about the change in the charge of an element or its component will pass through you, and all this will be difficult to assimilate.
12 Try not to get confused by the information. Information about different types of chemical reactions, about the exchange of electrons, about the change in the charge of an element or its component will pass through you, and all this will be difficult to assimilate. - Break difficult topics into chunks. For example, if you do not understand the oxidation reaction or the principle of combining elements with positive and negative charges, start speaking all the information you know, and you will understand that you have already managed to understand and remember a lot.
 13 Chat with your teacher regularly. Make a list of difficult topics and ask your teacher to help you. This will give you a chance to internalize the material before the group moves on to the next topic, which will further confuse you.
13 Chat with your teacher regularly. Make a list of difficult topics and ask your teacher to help you. This will give you a chance to internalize the material before the group moves on to the next topic, which will further confuse you.  14 Imagine chemistry is like learning a new language. It is important to understand that writing charges, the number of atoms in a molecule, and the bond between molecules is part of the language of chemistry. All this reflects what happens in nature on paper.
14 Imagine chemistry is like learning a new language. It is important to understand that writing charges, the number of atoms in a molecule, and the bond between molecules is part of the language of chemistry. All this reflects what happens in nature on paper. - It would be much easier to understand all this if all processes could be observed live. It is important for you not only to understand the principles of the processes, but also the language that is used to record this information.
- If you find it difficult to study chemistry, remember that you are alone and don't give up. Talk to your instructor, the group, or anyone who is well versed in the subject. All this can be learned, but it would be more correct if someone could explain the material to you so that you understand everything.
Tips
- Don't forget to rest. Taking a break from your studies will allow you to return to school with a fresh mind.
- Get some sleep on the eve of the exam. A slept person has better memory and concentration.
- Reread what you already know. Chemistry is a science built on the study of one phenomenon and the expansion of knowledge. It is important to keep everything you have learned in memory so that the question on the exam does not surprise you.
- Get ready for class. Read all materials and do your homework. You will fall behind more and more if you miss something.
- Allocate time. Pay more attention to chemistry if this subject is not good for you, but do not devote all your time to it, because there are other subjects.