Author:
Marcus Baldwin
Date Of Creation:
13 June 2021
Update Date:
1 July 2024

Content
- Steps
- Method 1 of 3: How to Conduct a Basic Water Evaporation Experiment
- Method 2 of 3: How to make a distiller
- Method 3 of 3: How to Use Unusual Techniques
- Tips
- Warnings
How to get salt from sea water? For centuries, this question has puzzled sailors wandering the seas, and students, likewise, wandering around science fairs. The answer is simple: evaporation. When you force seawater to evaporate (naturally or by heating it artificially), only the water is converted into steam, and the salt remains. With this knowledge, it is fairly easy to separate salt from water using simple materials that you may already have in your home.
Steps
Method 1 of 3: How to Conduct a Basic Water Evaporation Experiment
 1 Heat water and add salt to it to make salt water. With this simple experiment, it's easy to see the principles of evaporation in action. To get started, you need regular fine table salt, tap water, a frying pan, some black cardboard and a stove. Pour a few cups of water into a skillet and place it on a lit burner. Wait until the water heats up: it doesn't have to boil, it's just that the hotter it is, the faster the salt will dissolve in it.
1 Heat water and add salt to it to make salt water. With this simple experiment, it's easy to see the principles of evaporation in action. To get started, you need regular fine table salt, tap water, a frying pan, some black cardboard and a stove. Pour a few cups of water into a skillet and place it on a lit burner. Wait until the water heats up: it doesn't have to boil, it's just that the hotter it is, the faster the salt will dissolve in it. - The reason hot water is more suitable for dissolving salt (and other chemicals) is due to the movement of the molecules that make it up. When the water heats up, its molecules move faster, bumping into the salt molecules and breaking the salt crystals apart.
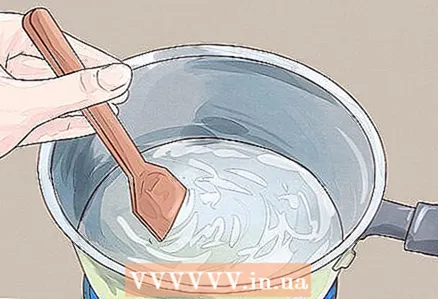 2 Add salt until it stops dissolving. Continue to pour it over a teaspoon and stir. Eventually, you will reach the state of water where it can no longer dissolve salt, no matter how hot it is. This is called a line saturation water. Turn off the burner and let the water cool slightly.
2 Add salt until it stops dissolving. Continue to pour it over a teaspoon and stir. Eventually, you will reach the state of water where it can no longer dissolve salt, no matter how hot it is. This is called a line saturation water. Turn off the burner and let the water cool slightly. - When water reaches the saturation line, it is no longer able to dissolve salt at the molecular level: the salt has already been dissolved so much that the water has no chemical potential to break new salt crystals.
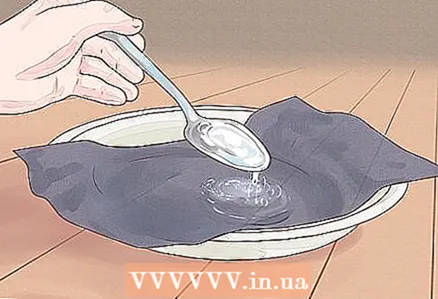 3 Spoon water over dark cardboard with a tablespoon. Using a scoop or tablespoon, pour some salt water onto a piece of dark cardboard. Place this piece on a plate ahead of time to avoid wetting your work surface or table. All you have to do now is wait for the water to evaporate. This process will be faster if the cardboard is left in direct sunlight.
3 Spoon water over dark cardboard with a tablespoon. Using a scoop or tablespoon, pour some salt water onto a piece of dark cardboard. Place this piece on a plate ahead of time to avoid wetting your work surface or table. All you have to do now is wait for the water to evaporate. This process will be faster if the cardboard is left in direct sunlight. - Don't throw away any leftover salt: there are thousands of things that can come in handy.For example, you can boil eggs in a bag, cook potatoes, canning spinach, and even peel nuts!
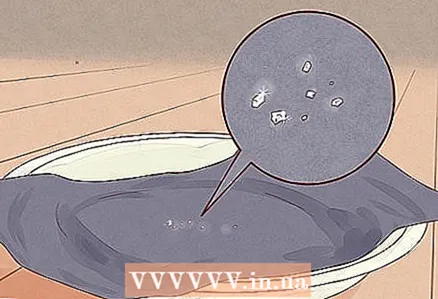 4 Wait for the salt to form. As it evaporates, the water will leave tiny salt crystals behind. They should appear as small shiny white or transparent flakes on the surface of the cardboard. Congratulations! You have just separated the salt from the water.
4 Wait for the salt to form. As it evaporates, the water will leave tiny salt crystals behind. They should appear as small shiny white or transparent flakes on the surface of the cardboard. Congratulations! You have just separated the salt from the water. - Calmly scrape some salt off the paper to season your food: it should be perfectly safe and edible. But be careful not to scrape off pieces of paper with it into your food!
Method 2 of 3: How to make a distiller
 1 Start by boiling a bucket of salt water. The simple experiment above shows you how to extract salt from water, but what if you want less salty water as well? Distillation is the answer. Distillation is the process of heating water to separate it from other dissolved chemicals, then collecting condensate, which must be relatively "clean". In this case, we'll start by making a few cups of salted water (read above for how) and boiling them on the stove.
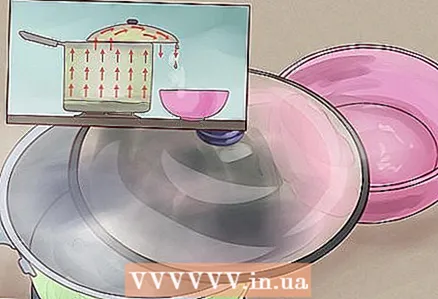
1 Start by boiling a bucket of salt water. The simple experiment above shows you how to extract salt from water, but what if you want less salty water as well? Distillation is the answer. Distillation is the process of heating water to separate it from other dissolved chemicals, then collecting condensate, which must be relatively "clean". In this case, we'll start by making a few cups of salted water (read above for how) and boiling them on the stove.  2 Cover the ladle with the lid, but not completely. Next, find a lid for your ladle (it doesn't have to fit perfectly). Place the lid so that some part of the lid hangs off the ladle and is below all other parts. Watch as condensation begins to form on the lid and then drips off.
2 Cover the ladle with the lid, but not completely. Next, find a lid for your ladle (it doesn't have to fit perfectly). Place the lid so that some part of the lid hangs off the ladle and is below all other parts. Watch as condensation begins to form on the lid and then drips off. - As the salt water boils, the water itself (no salt) will turn into steam and rise from the ladle. When hitting the lid, the steam will cool slightly and form liquid condensation (water) on the bottom of the lid. This water does not contain salt, so all we have to do is collect the salt-free water.
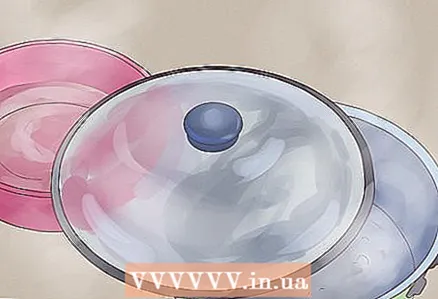
 3 Let the water accumulate in a bowl. As the water runs downward, condensation from the inside of the lid will naturally collect at its lowest point. As soon as there is enough of it, it will begin to form into drops and fall down. Place a bowl under this point to catch drops of distilled water.
3 Let the water accumulate in a bowl. As the water runs downward, condensation from the inside of the lid will naturally collect at its lowest point. As soon as there is enough of it, it will begin to form into drops and fall down. Place a bowl under this point to catch drops of distilled water. - If you want, you can lower a long, narrow metal or glass object (like a glass stirring rod or thermometer) from the bottom of the lid into the bowl and water will run down it straight into the container.
 4 Repeat the previous step if necessary. The longer the water in the ladle boils, the more distilled water must collect in the bowl. This water will be stripped of most of the salt. However, in some cases, a small amount of salt will still remain. Then you may need double distillation: boiling water already collected in a bowl to remove salt residues.
4 Repeat the previous step if necessary. The longer the water in the ladle boils, the more distilled water must collect in the bowl. This water will be stripped of most of the salt. However, in some cases, a small amount of salt will still remain. Then you may need double distillation: boiling water already collected in a bowl to remove salt residues. - Technically, this water should be drinkable. However, if you are not sure that the lid of the ladle and the bowl for collecting water (and a metal or glass rod to drain it, if you used one) are clean, you should not drink it.
Method 3 of 3: How to Use Unusual Techniques
 1 Use reverse osmosis. The methods described above are far from the only ones for separating salt from water, they are just the most convenient for most people at home. But you can still purify water from salt using special materials. For example, a process called reverse osmosis can remove salt from water through a permeable membrane. This membrane acts as a filter, allowing only water molecules to pass through and trapping dissolved contaminants like salt.
1 Use reverse osmosis. The methods described above are far from the only ones for separating salt from water, they are just the most convenient for most people at home. But you can still purify water from salt using special materials. For example, a process called reverse osmosis can remove salt from water through a permeable membrane. This membrane acts as a filter, allowing only water molecules to pass through and trapping dissolved contaminants like salt. - Reverse osmosis pumps are sometimes sold for home use, but they are also often used on vacation, such as camping trips. These pumps can be expensive, typically costing several hundred dollars.
 2 Add decanoic acid. Another way to separate salt from water is by chemical reaction.Research has shown, for example, that treating salt water with a chemical called decanoic acid is a reliable way to remove salt. After adding acid and light heating and cooling, salt and other impurities "fall out" from the solution (that is, they solidify and settle at the bottom). When the reaction is complete, the water and salt are in two completely separate layers, making it so easy to separate the water.
2 Add decanoic acid. Another way to separate salt from water is by chemical reaction.Research has shown, for example, that treating salt water with a chemical called decanoic acid is a reliable way to remove salt. After adding acid and light heating and cooling, salt and other impurities "fall out" from the solution (that is, they solidify and settle at the bottom). When the reaction is complete, the water and salt are in two completely separate layers, making it so easy to separate the water. - Decanoic acid is available at chemical stores and typically costs around $ 30-40 per bottle.
 3 Use electrodialysis. Using the force of electricity, it is possible to remove particles like salt from the water. This is done by immersing the negatively charged anode and the positively charged cathode in water and separating them with a porous membrane. The electrical charges of the anode and cathode attract dissolved ions (for example, those that make up salt) like magnets, leaving relatively clear water.
3 Use electrodialysis. Using the force of electricity, it is possible to remove particles like salt from the water. This is done by immersing the negatively charged anode and the positively charged cathode in water and separating them with a porous membrane. The electrical charges of the anode and cathode attract dissolved ions (for example, those that make up salt) like magnets, leaving relatively clear water. - Note that bacteria and other contaminants are not removed from the water as a result of this procedure, so further purification will be required to obtain potable water. However, thanks to recent research, new techniques have emerged that really kill bacteria in the process.
Tips
- Don't use salt water unless you have other options. In addition to salt, it contains minerals, organic matter and other contaminants that are difficult to completely cleanse of salt.
Warnings
- Be careful when boiling water on the stove. If you need to touch hot dishes, be sure to use oven mitts or a towel to protect yourself from the heat.
- Don't drink salt water when lost in the wild. Our bodies need even more water to get rid of the excess salt that comes with it, so salt water dehydrates us even more.