Author:
Randy Alexander
Date Of Creation:
1 April 2021
Update Date:
1 July 2024

Content
In chemistry, valence electrons are electrons located in the outermost layer of the electron shell of an element. Determining the number of valence electrons of an element is an important skill in chemistry because this information will help determine the types of bonds that element can form. Determining the number of valence electrons can be easily done with the periodic table of chemical elements.
Steps
Part 1 of 2: Find the number of valence electrons using the periodic table
With non-transition metal
Have one ready periodic table chemical elements. The periodic table of elements (the periodic table for short) is a color-coded multi-cell table that lists all the known elements as well as some essential information about those elements. Based on the information available in the periodic table, we can determine the number of valence electrons of the element we are investigating. The periodic table is usually attached to a textbook. You can also refer to this existing interactive periodic table.

Number each column in the periodic table from 1 to 18. Usually in the periodic table, all elements in the same column will have the same number of valence electrons. If your periodic table hasn't numbered columns yet, do it yourself by numbering 1 through 18 vertically from left to right. Scientifically, each column in the periodic table is called one "group".- For example, for an unsigned periodic table, we would number 1 above the element Hydrogen (H), the number 2 above the element Beri (Be) and do the same until 18 above Helium (He ).

Determine the position of the element in question. In this step determine the position of the element you are looking at on the periodic table. You can find the position of an element based on its chemical symbol (letter in each cell), atomic number (the number in the upper left corner of each cell), or based on information messages are available on the periodic table.- For example we need to find the number of valence electrons of the element Carbon (C). The element's atomic number is 6. The carbon is in the upper part of the group 14 elements. In the next step we will determine the number of valence electrons of this element.
- In this section we will ignore Transition Metals, ie elements in the range of groups 3 to 12. These transition metals are slightly different from the rest, so the steps are The instructions given in this section do not apply to such metals. We will look at these groups of elements later in the article.

Use the group number to determine the number of valence electrons. The group number of a non-transition metal can be used to calculate the number of valence electrons in that element's atom. The "unit row of group number" is the number of valence electrons in the atoms of the elements in that group. In other words:- Group 1: 1 valence electron
- Group 2: 2 valence electrons
- Group 13: 3 valence electrons
- Group 14: 4 valence electrons
- Group 15: 5 valence electrons
- Group 16: 6 valence electrons
- Group 17: 7 valence electrons
- Group 18: 8 valence electrons (except for helium with 2 valence electrons)
- In the carbon example, since the carbon is in group 14, we could say that a carbon atom has four valence electrons.
With transition metal
Identify an element in the range from Group 3 to Group 12. As mentioned above, the elements in groups 3 to 12 are called "transition metals" and when it comes to valence electrons these have different properties from the rest. In this section, we will learn why it is often not possible to assign valence electrons to the atoms of transition metals.
- In this section we take the element Tantan (Ta) whose atomic number is 73 as an example. The next steps will help determine the element's number of valence electrons.
- Note that the elements of the 3 family lantans and actinium (also known as the "rare earth metals") also belong to the group of transition metals - these two groups of elements are usually listed below the periodic table. head with lantan and actini.
Valence electrons in transition metals are not the same as' normal 'valence electrons'. To understand why transition metals don't actually '' work '' like other elements on the periodic table, we need to know a little bit about how electrons work in the atom as explained below. , or you can skip this step.
- When electrons are inserted into an atom, they are arranged into different "orbitals" - that is, different regions around the nucleus. In short, valence electrons are the electrons located in the outermost layer orbital - in other words, the last electrons added to the atom.
- Explaining the orbital in detail is perhaps a bit complicated, when electrons are added to the subclass d of the atomic shell of the transition metal (see below), the first of these electrons will behave like conventional valence electrons, but then their properties can change, double when electrons from other orbitals can act as valence electrons. That is, an atom can have multiple valence electrons depending on the case.
- You can learn more about this at the Clackamas Community College valence electron site.
Determine the number of valence electrons based on the group number. As noted above for non-transition metals, the group number on the periodic table can help determine the number of valence electrons. However, there is no definite formula to determine the exact number of valence electrons of the transition metal - in this case, the number of valence electrons of an element is not at a fixed value, the number of things. self groups can only tell a relative number of valence electrons. Detail:
- Group 3: 3 valence electrons
- Groups of 4: 2 to 4 valence electrons
- Group 5: 2 to 5 valence electrons
- Group 6: 2 to 6 valence electrons
- Groups 7: 2 to 7 valence electrons
- Groups of 8: 2 to 3 valence electrons
- Groups 9: 2 to 3 valence electrons
- Groups of 10: 2 to 3 valence electrons
- Groups 11: 1 to 2 valence electrons
- Group 12: 2 valence electrons
- Taking the example of the element Tanta (Ta) of group 5, we can say that this element has from 2 to 5 valence electrons, depending on the case.
Part 2 of 2: Find the number of valence electrons based on electron configuration
Learn how to read electron configuration. Based on the electron configuration of an element, we can also determine the number of valence electrons of that element. Electron configuration looks complicated, but it is just how to represent the orbitals of an element in the form of letters and numbers, once you have grasped the law, understanding electron configuration is not difficult.
- Consider an example electron configuration of sodium (Na):
- 1s2s2p3s
- If you pay attention, you will see that the electron configuration is just a string of repeats:
- (number) (word) (number) (word) ...
- ... and so on. Group (number) (word) the first is the name of the orbital and denotes the number of electrons in that orbital.
- So, in our case, we can say that sodium does 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, 6 electrons in a 2p orbital and 1 electron in 3 3s orbital. There are 11 electrons in total - sodium's atomic number is also 11.
- Consider an example electron configuration of sodium (Na):
Find the electron configuration of the element you're looking at. Once you know the electron configuration of an element, finding the electron configuration of that element is not difficult (except in the case of transition metals). If the electron configuration is available in the question you need to solve, you can skip this step. If you need to find the electron configuration, proceed with the following steps:
- The complete electron configuration of the element ununocti (Uuo), the atomic number 118 is:
- 1s2s2p3s3p4s3d4p5s4d5p6s4f5d6p7s5f6d7p
- Once you have such a complete electron configuration, to find the electron configuration of another element, you just need to fill the orbitals with electrons, starting with the first orbital, until the number of electrons has run out to fill. It sounds complicated, but when it comes to doing it it's relatively easy. For example, if we wanted to write the complete electron configuration of chlorine (Cl), element 17, that is, this element's atom has 17 electrons, we would fill in the following:
- 1s2s2p3s3p
- Note that the total number of electrons in electron configuration is just right 17: 2 + 2 + 6 + 2 + 5 = 17. You just need to change the number on the last orbital - the rest remains the same because the near-penultimate orbital is full. electron.
- Learn more about how to write an element's electron configuration.
- The complete electron configuration of the element ununocti (Uuo), the atomic number 118 is:

Assign electrons to orbitals according to the Eighth Rule. When electrons are added to an atom, they are sorted into orbitals in the order stated above - the first two electrons will be placed in the 1s orbital, the next two electrons in the 2s orbital, the next six electrons are placed in the orbital 2p, do so until the electron is placed in the corresponding orbital. When we consider the atoms of non-transition elements, we can say that these orbitals will form "layers" around the nucleus, in which the following layer will be further away from the nucleus than the one before it. In addition to the first orbital layer which can only hold up to two electrons, all subsequent orbital layers can hold up to eight electrons (except in the case of transition metals). This rule is called The Eightfold Rule.- For example, consider the element Bo (B). The atomic number of this element is 5, so we have the electron configuration of this element as follows: 1s2s2p. Since the first orbital shell contains only 2 electrons, it is possible to determine that Bo has two orbital layers: the first one consisting of 2 electrons at the 1s orbital and the second with three electrons distributed in the 2s and 2p orbitals. .
- For another example, an element similar to chlorine would have three layers: a layer of two electrons in the 1s orbital, a layer of two electrons in the 2s orbital and six electrons in the 2p orbital, and an outer layer of two electrons in the 3 orbital and five electrons in a 3p orbital.
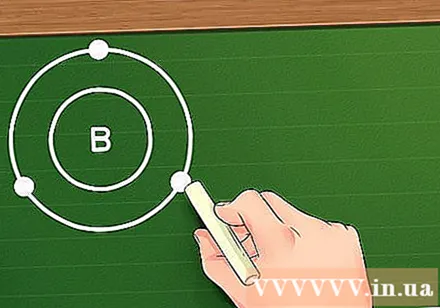
Find the number of electrons in the outermost layer. Once the electron configuration has been determined, we already know the layers of that element, finding the number of valence electrons can be done by determining the number of electrons in the outermost layer of the atomic electron shell. If the outermost layer is full (ie already with a total of eight electrons, or for the first layer 2 electrons) then that element is called an inert element and is hardly involved in chemical reactions. However, this rule does not apply to transition metals.- For example Bo, since Bo has three electrons in the second layer, also the outermost layer, so we can say that element Bo has father valence electrons.

Use the row number on the periodic table as an abbreviated way to determine the number of orbital layers. The horizontal row on the periodic table is called "cycle" of the elements. Starting from the first row, each cycle corresponds to the 'number of electron layers' of the elements in the same period. Therefore, you can use the period to quickly determine the number of valence electrons of an element - you just count the number of electrons in order from left to right from the first element of that period. Note once again that this is not applicable to transition metals.- For example, since selenium belongs to cycle 4, it can be determined that the element has four electron layers in the atomic shell. Since in order from left to right, this is the sixth element in cycle 4 (excluding the transition metal), we can say that the fourth shell of selenium has six electrons, i.e. this element has six valence electrons.
Advice
- Note, the electron configuration can be briefly written using rare gases (elements of group 18) instead of orbitals at the top of the configuration. For example, the electron configuration of sodium could be written as 3s1 - that is, the electron configuration of sodium is the same as that of Neon but there is an extra electron in the 3s orbital.
- Transition metals may have incomplete valence subclasses. In order to accurately determine the valence number of the transition metal, it is necessary to apply complex quantum principles that are not covered by this article.
- It is also important to note that the periodic table of chemical elements can be different in different countries. So, make sure you are using the common periodic table where you live to avoid confusion.
What you need
- Periodic table of chemical elements
- Pencil
- Paper