Author:
Robert Simon
Date Of Creation:
24 June 2021
Update Date:
1 July 2024

Content
- To step
- Part 1 of 2: Determining the limiting reagent
- Part 2 of 2: Determining the theoretical yield
The theoretical yield is a term used in chemistry for the maximum amount of a substance that you expect from a chemical reaction. You start by balancing a reaction equation and defining the limiting reagent. When you measure the amount of reagent you want to use, you can calculate the amount of a substance obtained. This is the theoretical yield of the equation. In an actual experiment, you will likely lose some of it, because it is not an ideal experiment.
To step
Part 1 of 2: Determining the limiting reagent
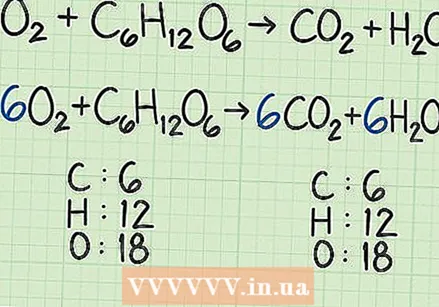 Start with an equilibrium reaction. A reaction equation is similar to a recipe. It shows which reagents (on the left) react with each other to form products (on the right). An equilibrium reaction will have the same number of atoms on the left side of the equation (as reactants) as on the right side (in the form of products).
Start with an equilibrium reaction. A reaction equation is similar to a recipe. It shows which reagents (on the left) react with each other to form products (on the right). An equilibrium reaction will have the same number of atoms on the left side of the equation (as reactants) as on the right side (in the form of products). - For example, let's say we have the simple equation
 Calculate the molar mass of each reaction. Using the periodic table or some other reference book, look up the molar mass of each atom in each composition. Add them together to find the molar mass of each compound of reagents. Do this for a single molecule of the compound. Consider again the equation of the conversion of oxygen and glucose into carbon dioxide and water:
Calculate the molar mass of each reaction. Using the periodic table or some other reference book, look up the molar mass of each atom in each composition. Add them together to find the molar mass of each compound of reagents. Do this for a single molecule of the compound. Consider again the equation of the conversion of oxygen and glucose into carbon dioxide and water: 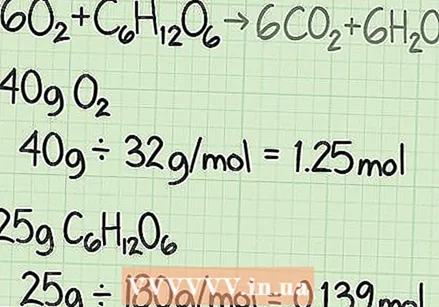 Convert the amount of each reagent from grams to moles. For a real experiment, the mass in grams of each reagent you use will be known. Divide this value by the molar mass of that substance in conversion to the number of moles.
Convert the amount of each reagent from grams to moles. For a real experiment, the mass in grams of each reagent you use will be known. Divide this value by the molar mass of that substance in conversion to the number of moles. - For example, suppose you start with 40 grams of oxygen and 25 grams of glucose.
- 40 g
 Determine the molar ratio of the reagents. A mole is a calculation tool used in chemistry for counting molecules based on their mass. By determining the number of moles of both oxygen and glucose, you know how many molecules of each you start with. To find the ratio of both, divide the number of moles of one reagent by those of the other.
Determine the molar ratio of the reagents. A mole is a calculation tool used in chemistry for counting molecules based on their mass. By determining the number of moles of both oxygen and glucose, you know how many molecules of each you start with. To find the ratio of both, divide the number of moles of one reagent by those of the other. - In the following example, you start with 1.25 moles of oxygen and 0.139 moles of glucose. So the ratio of oxygen and glucose molecules is 1.25 / 0.139 = 9.0. This ratio means that you have nine times as many molecules of oxygen as glucose.
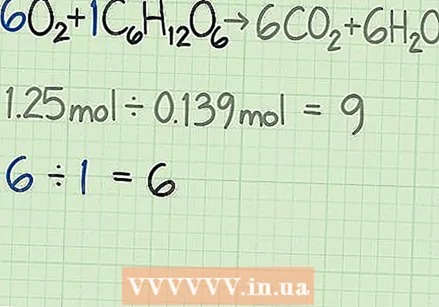 Determine the ideal ratio for the reaction. Look at the equilibrium response. The coefficients for each molecule tell you the ratio of the molecules you need for the reaction to occur. If you are using exactly the ratio given by the formula, then both reagents should be used equally.
Determine the ideal ratio for the reaction. Look at the equilibrium response. The coefficients for each molecule tell you the ratio of the molecules you need for the reaction to occur. If you are using exactly the ratio given by the formula, then both reagents should be used equally. - For this reaction the reactants are given as
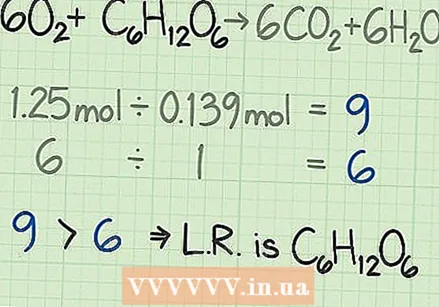 Compare the ratios to find the limiting reagent. In most chemical reactions, one of the reagents will be used up earlier than the other. The reagent that is used up first is called the limiting reagent. This limiting reagent determines how long the chemical reaction can continue and the theoretical yield you can expect. Compare the two ratios you calculated to determine the limiting reagent:
Compare the ratios to find the limiting reagent. In most chemical reactions, one of the reagents will be used up earlier than the other. The reagent that is used up first is called the limiting reagent. This limiting reagent determines how long the chemical reaction can continue and the theoretical yield you can expect. Compare the two ratios you calculated to determine the limiting reagent: - In the following example, you start with nine times as much oxygen as glucose, measured by moles. The formula tells you that your ideal ratio is six times more oxygen to glucose. So you need more oxygen than glucose. So the other reagent, glucose in this case, is the limiting reagent.
- For this reaction the reactants are given as
- For example, let's say we have the simple equation
Part 2 of 2: Determining the theoretical yield
 View the response to find the product you want. The right side of a chemical equation shows the products that the reaction yields. When the reaction is balanced, the coefficients of each product indicate how many of each molecular ratios you can expect. Each product has a theoretical yield, or the amount of product that you would expect when the reaction is completely complete.
View the response to find the product you want. The right side of a chemical equation shows the products that the reaction yields. When the reaction is balanced, the coefficients of each product indicate how many of each molecular ratios you can expect. Each product has a theoretical yield, or the amount of product that you would expect when the reaction is completely complete. - Continuing with the example above, you analyze the response
 Record the number of moles of your limiting reagent. You should always compare the number of moles of limiting reagent with the number of moles of a product. If you try to compare the masses of each, you will not get the correct result.
Record the number of moles of your limiting reagent. You should always compare the number of moles of limiting reagent with the number of moles of a product. If you try to compare the masses of each, you will not get the correct result. - In the example above, glucose is the limiting reagent. According to molar mass calculations, the first 25 g of glucose equals 0.139 mole of glucose.
 Compare the ratio between the molecules in the product and the reagent. Return to the equilibrium reaction. Divide the number of molecules of your desired product by the number of molecules of your limiting reagent.
Compare the ratio between the molecules in the product and the reagent. Return to the equilibrium reaction. Divide the number of molecules of your desired product by the number of molecules of your limiting reagent. - The equilibrium reaction for this example is
 Multiply this ratio by the number of moles of the limiting reagent. The answer is the theoretical yield, in moles, of the desired product.
Multiply this ratio by the number of moles of the limiting reagent. The answer is the theoretical yield, in moles, of the desired product. - In this example, the 25 g of glucose equals 0.139 moles of glucose. The ratio of carbon dioxide and glucose is 6: 1. You expect to be able to produce six times as many moles of carbon dioxide as the number of moles of glucose you started with.
- The theoretical yield of carbon dioxide is (0.139 mol glucose) x (6 mol carbon dioxide / mol glucose) = 0.834 mol carbon dioxide.
 Convert the result to grams. This is the reverse of your previous step of calculating the number of moles or the amount of reagent. When you know the number of moles you can expect, multiply that by the molar mass of the product to get the theoretical yield in grams.
Convert the result to grams. This is the reverse of your previous step of calculating the number of moles or the amount of reagent. When you know the number of moles you can expect, multiply that by the molar mass of the product to get the theoretical yield in grams. - In the following example is the molar mass of CO2 about 44 g / mol. (The molar mass of carbon is ~ 12 g / mol and of oxygen ~ 16 g / mol, so the total is 12 + 16 + 16 = 44).
- Multiply 0.834 moles of CO2 x 44 g / mol CO2 = ~ 36.7 grams. The theoretical yield of the experiment is 36.7 grams of CO2.
 Repeat the calculation for the other product, if desired. In many experiments, you may only be interested in the yield of a particular product. If you want to know the theoretical yield of both products, all you have to do is repeat the process.
Repeat the calculation for the other product, if desired. In many experiments, you may only be interested in the yield of a particular product. If you want to know the theoretical yield of both products, all you have to do is repeat the process. - In this example, water is the second product
. According to the equilibrium reaction, you can expect six molecules of water from one molecule of glucose. This is a ratio of 6: 1. So 0.139 moles of glucose should result in 0.834 moles of water.
- Multiply the number of moles of water by the molar mass of water. The molar mass is 2 + 16 = 18 g / mol. Multiplied by the product, this results in 0.139 mol H2O x 18 g / mol H2O = ~ 2.50 grams. The theoretical yield of water in this experiment is 2.50 grams.
- In this example, water is the second product
- The equilibrium reaction for this example is
- Continuing with the example above, you analyze the response