Author:
Frank Hunt
Date Of Creation:
16 March 2021
Update Date:
27 June 2024

Content
- To step
- Method 1 of 2: Using quick rules
- Method 2 of 2: Calculation of the solubility of the K.sp
- Necessities
- Tips
- Warnings
In chemistry, solubility is used to describe the properties of a solid that is mixed with and completely dissolves in a liquid, without leaving undissolved particles. Only (charged) ionic compounds are soluble. For practical purposes, memorizing a few rules, or referring to a list of rules, is enough to tell you if most ionic compounds will remain solid when mixed with water, or if a significant amount will dissolve. In reality, some molecules will dissolve even if you don't see any changes, so for precise experiments you will need to know how to calculate this amount.
To step
Method 1 of 2: Using quick rules
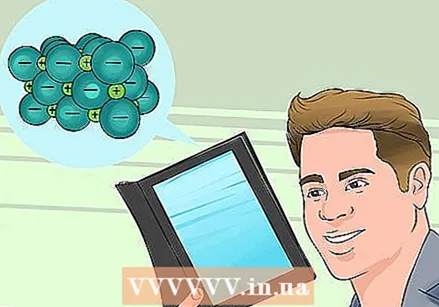 Know more about ionic compounds. Each atom normally has a number of electrons, but sometimes they gain or lose one extra electron. The result is one ion with an electric charge. When an ion with a negative charge (an extra electron) meets an ion with a positive charge (an electron is missing), they bond together, just like the negative and positive ends of two magnets. The result is an ionic bond.
Know more about ionic compounds. Each atom normally has a number of electrons, but sometimes they gain or lose one extra electron. The result is one ion with an electric charge. When an ion with a negative charge (an extra electron) meets an ion with a positive charge (an electron is missing), they bond together, just like the negative and positive ends of two magnets. The result is an ionic bond. - Ions with a negative charge are called anions, and ions with a positive charge cations.
- Normally, the number of electrons in an atom is equal to the number of protons, where the electric charges are in equilibrium.
 Know solubility. Water molecules (H.2O) have an unusual structure, with which they behave like a magnet: one end has a positive charge while the other end is negatively charged. When you mix an ionic bond with water, these "water magnets" will gather around it, trying to pull the positive and negative ions apart. Some ionic bonds are not very tight together; these are solublebecause water will tear and dissolve the bond. Other composites have stronger bonds, and are Not solvablebecause they can stick together despite the water molecules.
Know solubility. Water molecules (H.2O) have an unusual structure, with which they behave like a magnet: one end has a positive charge while the other end is negatively charged. When you mix an ionic bond with water, these "water magnets" will gather around it, trying to pull the positive and negative ions apart. Some ionic bonds are not very tight together; these are solublebecause water will tear and dissolve the bond. Other composites have stronger bonds, and are Not solvablebecause they can stick together despite the water molecules. - Some connections have internal bonds that are comparable in strength to the pull of the water. These substances are moderately soluble, because a significant portion (but not all) of the bonds will be pulled apart.
 Study the rules of solubility. Since the interactions between atoms are quite complex, it is not always intuitive which compounds are soluble and insoluble. Find the first ion in the compound in the list below to find out how it usually behaves, then check the exceptions to make sure the second ion doesn't interact abnormally.
Study the rules of solubility. Since the interactions between atoms are quite complex, it is not always intuitive which compounds are soluble and insoluble. Find the first ion in the compound in the list below to find out how it usually behaves, then check the exceptions to make sure the second ion doesn't interact abnormally. - For example, to use strontium chloride (SrCl2), search for Sr or Cl in the bold steps indicated below. Cl is "mostly solvable" so check for exceptions below. Sr is not indicated as an exception, so SrCl2 be soluble.
- The most common exceptions to each rule are listed below. There are other exceptions, but you probably won't find them in a common chemistry class or lab.
 Compounds are soluble when they contain alkali metals, including Li, Na, K, Rb and Cs. These are also called the elements of Group IA: lithium, sodium, potassium, rubidium and cesium. Almost any compound with any of these ions is soluble.
Compounds are soluble when they contain alkali metals, including Li, Na, K, Rb and Cs. These are also called the elements of Group IA: lithium, sodium, potassium, rubidium and cesium. Almost any compound with any of these ions is soluble. - Exception: Li3PO4 is not soluble.
 Compounds with NO3, C2H.3O2, NO2, ClO3 and ClO4 are soluble. These are nitrate, acetate, nitrite, chlorate and perchlorate ions respectively. Note that acetate is often abbreviated with OAc.
Compounds with NO3, C2H.3O2, NO2, ClO3 and ClO4 are soluble. These are nitrate, acetate, nitrite, chlorate and perchlorate ions respectively. Note that acetate is often abbreviated with OAc. - Exceptions: Ag (OAc) (silver acetate) and Hg (OAc)2 (mercury acetate) are not soluble.
- AgNO2 and KClO4 are only "partially soluble".
 compounds with Cl, Br and I are usually soluble. Chloride, bromide and iodide ions almost always form soluble compounds, also known as the halogen salts.
compounds with Cl, Br and I are usually soluble. Chloride, bromide and iodide ions almost always form soluble compounds, also known as the halogen salts. - Exception: If either of these binds with ions of silver (Ag), mercury (Hg2), or lead (Pb), the result is not soluble. The same applies to the less common compounds with copper (Cu) and thallium (Tl).
 Connections to SO4 are usually soluble. The sulfate ion usually forms soluble compounds, but there are several exceptions.
Connections to SO4 are usually soluble. The sulfate ion usually forms soluble compounds, but there are several exceptions. - Exceptions: The sulfate ion forms insoluble compounds with the following ions: strontium Sr, barium Ba, lead Pb, silver Ag, calcium Ca, radium Ra and diatomic silver Ag2. Note that silver sulfate and calcium sulfate dissolve just enough to be sometimes called sparingly soluble.
 Compounds with OH or S are not soluble. These are the hydroxide and sulfide ions, respectively.
Compounds with OH or S are not soluble. These are the hydroxide and sulfide ions, respectively. - Exceptions: Do you remember the alkali metals (Group I-A) and how much they like to form insoluble compounds? Li, Na, K, Rb and Cs all form soluble compounds with hydroxide or sulfide ions. In addition, hydroxide forms soluble salts with alkaline earth metals (Group II-A) ions: calcium Ca, strontium Sr and barium Ba. Note that the hydroxide with alkaline earth compound has just enough molecules to stick together to be sometimes considered "sparingly soluble".
 Compounds with CO3 or PO4 are not soluble. Check one last time for carbonate and phosphate ions, and you should know what to expect from the compound.
Compounds with CO3 or PO4 are not soluble. Check one last time for carbonate and phosphate ions, and you should know what to expect from the compound. - Exceptions: These ions form soluble compounds with the usual substances, the alkali metals Li, Na, K, Rb and Cs, as well as with ammonium NH4.
Method 2 of 2: Calculation of the solubility of the K.sp
 Look up the solubility product of the constant K.sp. This constant is different for each connection, so you will need to look it up in a table in your textbook or online. Since these values are determined experimentally, they can vary greatly from table to table, so it is best to use the table in your textbook, if there is one. Unless otherwise stated, most tables assume an ambient temperature of 25o C.
Look up the solubility product of the constant K.sp. This constant is different for each connection, so you will need to look it up in a table in your textbook or online. Since these values are determined experimentally, they can vary greatly from table to table, so it is best to use the table in your textbook, if there is one. Unless otherwise stated, most tables assume an ambient temperature of 25o C. - For example, if you want to dissolve lead iodide (PbI2), write down the equilibrium constant of the solubility product. If you are using a table on bilbo.chm.uri.edu, use the constant 7.1 × 10.
 First, write down the chemical equation. First, determine how the compound breaks down into ions when it dissolves. Now write an equation with K.sp on the one hand and the individual ions on the other.
First, write down the chemical equation. First, determine how the compound breaks down into ions when it dissolves. Now write an equation with K.sp on the one hand and the individual ions on the other. - For example, a molecule of PbI2 splits into the ions Pb, I and another I (you only need to know or look up the charge of one ion, because you know that the total compound always has a neutral charge).
- Write the equation 7.1 × 10 = [Pb] [I]
 Adjust the equation to use variables. Rewrite the equation as a single algebra problem, using your knowledge of the number of molecules or ions. Set x equal to the amount of the substance that will dissolve, and rewrite the variables as the numbers of each ion in terms of x.
Adjust the equation to use variables. Rewrite the equation as a single algebra problem, using your knowledge of the number of molecules or ions. Set x equal to the amount of the substance that will dissolve, and rewrite the variables as the numbers of each ion in terms of x. - In our example, we rewrite 7.1 × 10 = [Pb] [I]
- Since there is only one lead ion (Pb) in the compound, the number of dissolved compound molecules will be equal to the number of free lead ions. So we can replace [Pb] with x.
- Since there are two iodine ions (I) for each lead ion, we can equate the number of iodine atoms to 2x.
- The equation now reads 7.1 × 10 = (x) (2x)
 Consider common ions, if any. Skip this step if you are dissolving the compound in pure water. However, if the compound is dissolved in a solution that already contains one or more of the constituent ions (a "common ion"), the solubility is significantly reduced. The effect of the common ions is most noticeable in compounds that are usually insoluble, and in these cases it can be assumed that the vast majority of ions at equilibrium come from the ion already present in the solution. Rewrite the equation with the known molar concentration (moles per liter, or M) of the ions already in the solution, replacing the value of x you used for that ion.
Consider common ions, if any. Skip this step if you are dissolving the compound in pure water. However, if the compound is dissolved in a solution that already contains one or more of the constituent ions (a "common ion"), the solubility is significantly reduced. The effect of the common ions is most noticeable in compounds that are usually insoluble, and in these cases it can be assumed that the vast majority of ions at equilibrium come from the ion already present in the solution. Rewrite the equation with the known molar concentration (moles per liter, or M) of the ions already in the solution, replacing the value of x you used for that ion. - For example, if our lead-iodine compound were dissolved in a solution containing 0.2 M lead chloride (PbCl2), then we can rewrite the equation as 7.1 × 10 = (0.2M + x) (2x). And then, because 0.2M is such a higher concentration than x, we can safely rewrite this as 7.1 × 10 = (0.2M) (2x).
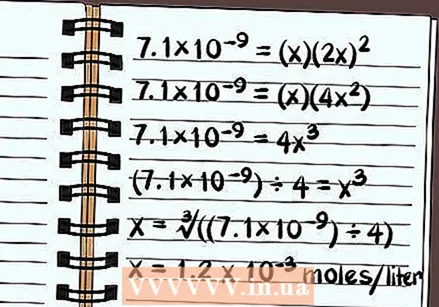 Solve the equation. Solve for x and know how soluble the compound is. Because of the way the solubility constant is defined, your answer will be expressed as the number of moles of the dissolved compound per liter of water. You may need a calculator to find the final answer.
Solve the equation. Solve for x and know how soluble the compound is. Because of the way the solubility constant is defined, your answer will be expressed as the number of moles of the dissolved compound per liter of water. You may need a calculator to find the final answer. - The following applies to solubility in pure water, not with any common ions.
- 7.1 × 10 = (x) (2x)
- 7.1 × 10 = (x) (4x)
- 7.1 × 10 = 4x
- (7.1 × 10) ÷ 4 = x
- x = ∛ ((7.1 × 10) ÷ 4)
- x = 1.2 x 10 moles per liter will dissolve. This is a very small amount, so you know that this compound is in principle poorly soluble.
Necessities
- Table of constants for solubility products (K.sp) for connections.
Tips
- If you have data from experiments about the degree to which a compound is dissolved, you can use the same equation to solve the solubility constant Ksp.
Warnings
- There is no universally accepted definition of these terms, but chemists agree on the majority of the compounds. Some marginal cases regarding the compounds with a significant proportion of dissolved and undissolved molecules can be described with different solubility tables.
- Some older textbooks give NH4OH again as a soluble composition. This is incorrect; small amounts of NH4 and OH ions can be observed, but cannot be isolated to form a compound.