Author:
Frank Hunt
Date Of Creation:
15 March 2021
Update Date:
1 July 2024

Content
Learn how to blow up a balloon using these common kitchen ingredients. Balloons that are inflated in this way are filled with carbon dioxide, which is created by the reaction of two ingredients. They don't contain helium, so they won't go up.
To step
Part 1 of 2: Blow up the balloon
 Pour a little vinegar into a plastic bottle. Choose a plastic water bottle or another bottle with a narrow neck. Pour 1 to 2 inches of vinegar into the bottle, using a funnel if you have one. Use white vinegar, also called distilled vinegar, for best results.
Pour a little vinegar into a plastic bottle. Choose a plastic water bottle or another bottle with a narrow neck. Pour 1 to 2 inches of vinegar into the bottle, using a funnel if you have one. Use white vinegar, also called distilled vinegar, for best results. - You can try this with any kind of vinegar, but it may take longer to blow up or require more vinegar to work. Other vinegars are also often more expensive.
- Vinegar can damage metal containers, potentially adding an unpleasant taste to food or drink if stored in that container. If you don't have plastic bottles, use a high-quality stainless steel bottle to minimize the chance. It can also help to weaken the vinegar with an equal amount of water, and it won't stop the balloon from getting blown up.
 Use a funnel or straw to put a little baking soda into an empty balloon. You can use any balloon shape and color. Hold it loosely by the nozzle, with the open side of the balloon facing you. If you have one, put a funnel in the nozzle, then pour about two tablespoons (30 ml) of baking soda into the balloon, or fill the balloon about half full.
Use a funnel or straw to put a little baking soda into an empty balloon. You can use any balloon shape and color. Hold it loosely by the nozzle, with the open side of the balloon facing you. If you have one, put a funnel in the nozzle, then pour about two tablespoons (30 ml) of baking soda into the balloon, or fill the balloon about half full. - If you don't have a funnel, you can stick a plastic straw into a pile of baking soda, hold your finger over the top opening of the straw, then insert the straw into the balloon and lift your finger. Tap the straw to let the baking soda fall out, then repeat until the balloon is at least 1/3 full.
 Stretch the balloon's nozzle over the top of the bottle. Be careful not to spill baking soda while doing this. Hold the nozzle of the balloon with both hands and stretch it over the opening of the plastic vinegar bottle. Have a friend hold the bottle steady if the table or bottle is shaky.
Stretch the balloon's nozzle over the top of the bottle. Be careful not to spill baking soda while doing this. Hold the nozzle of the balloon with both hands and stretch it over the opening of the plastic vinegar bottle. Have a friend hold the bottle steady if the table or bottle is shaky. 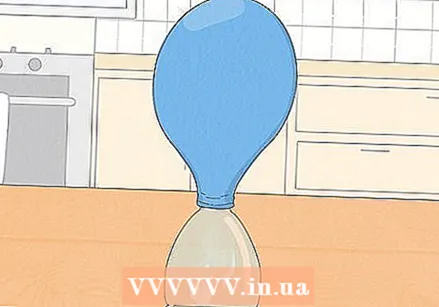 Lift the balloon over the bottle and watch the reaction. The baking soda should fall out of the balloon, through the neck of the bottle, and into the vinegar at the bottom. Here the two chemicals will hiss and react, turning into other chemicals. One of these is carbon dioxide, a gas that will pull up and inflate the balloon.
Lift the balloon over the bottle and watch the reaction. The baking soda should fall out of the balloon, through the neck of the bottle, and into the vinegar at the bottom. Here the two chemicals will hiss and react, turning into other chemicals. One of these is carbon dioxide, a gas that will pull up and inflate the balloon. - If there is not much sizzling, gently shake the bottle to mix the two ingredients.
 If it doesn't work, try again with more vinegar or baking soda. If the hissing has stopped and the balloon hasn't inflated after you count to 100, empty the bottle and try again with more vinegar and baking soda. The stuff left in the bottle has turned into other chemicals, mostly water, so it can't be reused.
If it doesn't work, try again with more vinegar or baking soda. If the hissing has stopped and the balloon hasn't inflated after you count to 100, empty the bottle and try again with more vinegar and baking soda. The stuff left in the bottle has turned into other chemicals, mostly water, so it can't be reused. - Do not exaggerate. There should never be more than 1/3 of the bottle filled with vinegar.
Part 2 of 2: How it works
 Understand chemical reactions. About everything around you is made up of molecules, or different types of substances. Often two kinds of molecules react with each other, fall apart and form other molecules from the pieces.
Understand chemical reactions. About everything around you is made up of molecules, or different types of substances. Often two kinds of molecules react with each other, fall apart and form other molecules from the pieces.  Learn about baking soda and vinegar. The reaction components, or substances that reacted with each other in the effervescent reaction you saw are baking soda and vinegar. Unlike many ingredients in your kitchen, these are both simple chemicals, not complicated mixtures of many chemicals:
Learn about baking soda and vinegar. The reaction components, or substances that reacted with each other in the effervescent reaction you saw are baking soda and vinegar. Unlike many ingredients in your kitchen, these are both simple chemicals, not complicated mixtures of many chemicals: - Baking soda is another word for the molecule sodium hydrogen carbonate.
- White vinegar is a mixture of this acetic acid and water. Only the acetic acid reacts with the baking soda.
 Read about the reaction. Baking soda is a type of substance that contains a base is called. Vinegar, or acetic acid, is a type of substance containing a acid is called. Bases and acids react with each other, partially breaking them down and forming different substances. This is described as "neutralization" because the end result is neither a base nor an acid. In this case, the new substances are water, a kind of salt and carbon dioxide. Carbon dioxide, a gas, exits the liquid mixture and expands throughout the bottle and balloon, inflating it.
Read about the reaction. Baking soda is a type of substance that contains a base is called. Vinegar, or acetic acid, is a type of substance containing a acid is called. Bases and acids react with each other, partially breaking them down and forming different substances. This is described as "neutralization" because the end result is neither a base nor an acid. In this case, the new substances are water, a kind of salt and carbon dioxide. Carbon dioxide, a gas, exits the liquid mixture and expands throughout the bottle and balloon, inflating it. - Although the definition of acid and base can be complicated, you can compare the differences between the original substances and the "neutralized" result to see that there are obvious changes. Vinegar, for example, has a strong odor and can be used to dissolve dirt. After mixed with baking soda, it smells much less and is no more effective with cleaning than water.
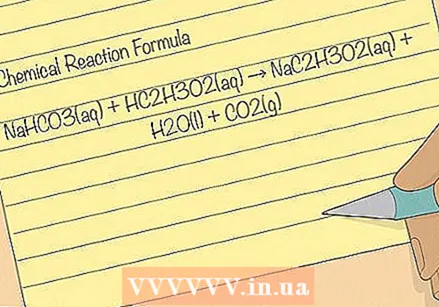 Study the chemical formula. If you are familiar with chemistry, or curious about how scientists describe reactions, the formula below describes the reaction between sodium hydrogen carbonate NaHCO3 and acetic acid HC2H.3O2(aq) NaC2H.3O2. Can you discover how each molecule splits and re-forms?
Study the chemical formula. If you are familiar with chemistry, or curious about how scientists describe reactions, the formula below describes the reaction between sodium hydrogen carbonate NaHCO3 and acetic acid HC2H.3O2(aq) NaC2H.3O2. Can you discover how each molecule splits and re-forms? - NaHCO3(w) + HC2H.3O2(w) → NaC2H.3O2(w) + H2O (v) + CO2(g)
- The letters in brackets show the state of chemicals during and after the reaction: (g) ash, (v) flaky or (w) aty. "Aqueous" means that the chemical is dissolved in water.
Tips
- This method can also be used in homemade cardboard or plastic rockets and you can take them very far if the ingredients are used in the right proportions. The reason it gets blown up is because the reaction creates gas and the pressure builds up.
Warnings
- If the balloon is fully inflated and the liquid is still bubbling, the balloon may explode. Decide if you have time to rip the balloon off or just cover your face before it gets splashed!
Necessities
- Balloon
- White vinegar
- Baking soda
- Narrow neck bottle
- Funnel (optional)