Author:
Randy Alexander
Date Of Creation:
25 April 2021
Update Date:
26 June 2024

Content
In chemistry, solubility is used to describe the properties of a solid compound when it is completely dissolved in a liquid without leaving any insoluble residue. Only ionic compounds (charged) are soluble. In fact, you only need to memorize a few principles or look up the literature to know whether an ionic compound will remain solid when added to water or if a large amount dissolves. Actually, a certain number of molecules will dissolve even if you don't see any change, so for the experiment to be accurate you have to know how to calculate the amount of this dissolved substance.
Steps
Method 1 of 2: Use quick rules
Learn about ionic compounds. Each atom usually has a certain number of electrons, but sometimes it gets or gives away an electron. This process makes it one ions charged. When an ion with a negative charge (excess of one electron) encounters an ion with a positive charge (missing an electron), they will bond together like the cathode and anode of two magnets. The result forms an ionic compound.
- Ions have a negative charge called anions, and ions have a positive charge called cation.
- Normally the number of electrons in an atom is equal to the number of protons, so it has no charge.
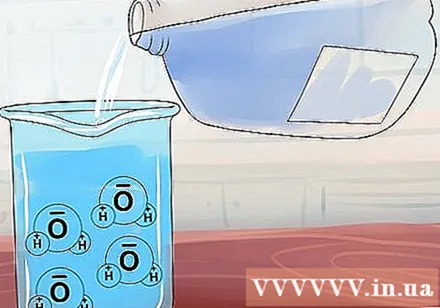
Understand solubility. Water molecule (H2O) has an irregular structure so it resembles a magnet: one end has a positive charge and the other has a negative charge. When you put an ionic compound in water, these water "magnets" gather around it, trying to pull the positive and negative ions apart.- Some ionic compounds are not very tightly absorbed, they are considered soluble Because it will separate and dissolve when added to water. Other compounds have stronger bonds insoluble because the ions are tightly attracted to each other regardless of the attraction of the water molecule.
- Some compounds have a binding force equivalent to the attraction of a water molecule. They are considered slightly soluble because most compounds will be separated, but the rest will still be attracted to each other.
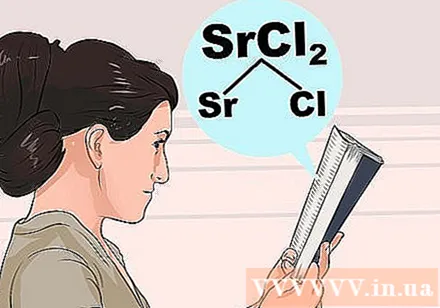
Understand the principle of dissolution. Because the interactions between atoms are so complex, you cannot rely entirely on intuition to distinguish which compounds can or cannot. Look up the first ion in the compound on the list below for its common properties, then check for exceptions to ensure that the second ion does not interact abnormally with it.- For example, to check strontium chloride (SrCl2), please look for Sr or Cl in the bold steps below. Cl is "usually soluble" so check for exceptions below it. Sr is not in the exception list so SrCl2 must be soluble.
- The most common exceptions to each rule are written below the rule. There are other exceptions, but these are unlikely to happen during normal chemistry or lab hours.
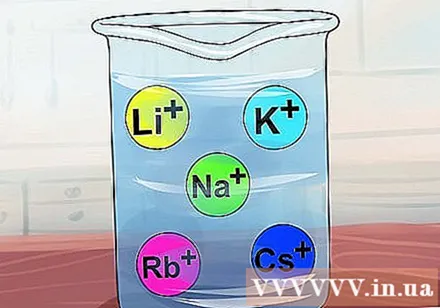
Compounds are soluble when they contain alkali metals such as Li, Na, K, Rb and Cs. These metals are also known as Group IA elements: lithium, sodium, potassium, rubidium and cesium. Almost all compounds containing one of these ions are soluble.- Exception: Li3PO4 indissoluble.
NO compounds3, C2H3O2, NO2, ClO3 and ClO4 are all soluble. The names corresponding to the above ions are nitrate, acetate, nitrite, chlorate and perchlorate. Note that acetate is often abbreviated as OAc.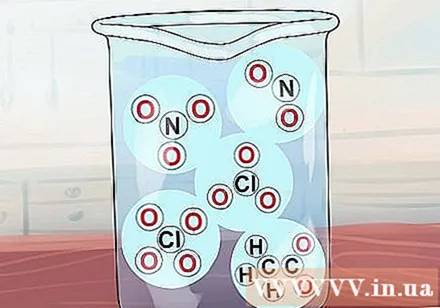
- Exception: Ag (OAc) (silver acetate) and Hg (OAc)2 (mercury acetate) insoluble.
- AgNO2 and KClO4 only "slightly melted".
The compounds of Cl, Br and I are usually soluble. Chloride, bromide and iodide ions almost always form soluble compounds, called halogen salts.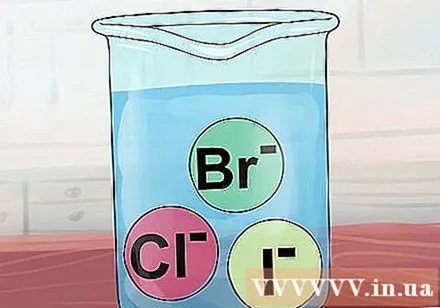
- Exception: If any of the above ions combine with silver ions Ag, mercury Hg2, or Pb lead, will form insoluble compounds. The same is true for the less common compounds formed when combined with copper Cu and thali Tl.
Compounds containing SO4 usually soluble. Sulfate ions usually form soluble compounds, but there are many exceptions.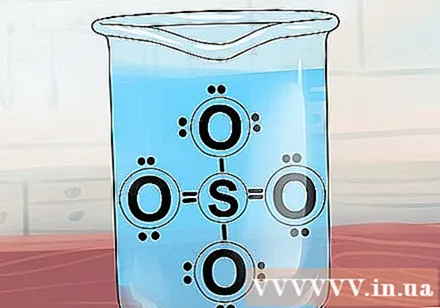
- Exception: Sulfate ions form an insoluble compound with the following ions: strontium Sr, barium Ba, lead Pb, silver Ag, calcium Ca, radium Ra, and silver monatom Ag2. Note that silver sulfate and calcium sulphate are only moderately soluble, so some consider them to be slightly soluble.
Substances containing OH or S are insoluble. The corresponding names for these ions are hydroxides and sulfides.
- Exception: Do you remember alkali metals (Groups I-A) and how they like to form soluble compounds? Li, Na, K, Rb and Cs all form compounds that are soluble with hydroxide or sulfide ions. In addition, hydroxides form salts that are soluble with alkaline earth metal ions (Group II-A): calcium Ca, strontium Sr, and barium Ba. Note: compounds made of hydroxides and alkaline earth metals actually have a significant number of molecules that remain bound together, so they are sometimes considered "slightly soluble".
CO-containing compounds3 or PO4 indissoluble. Check one last time for the carbonate and phosphate ions, and you will see if your compound is soluble.
- Exception: These ions form compounds that are soluble with alkali metals such as Li, Na, K, Rb and Cs, as well as with the ammonium ion NH4.
Method 2 of 2: Calculate the solubility from the constant Ksp
Look up the solubility product constant Ksp. This constant is different for each compound, so you should look it up on a graph in a textbook or online. Since these values are determined experimentally and can vary significantly between graphs, it is best to use the textbook's graph if available. Unless otherwise specified, most plots assume a test temperature of 25ºC.
- For example, let's say you are dissolving lead iodide with the formula PbI2, write its solubility product constant. If you refer to the graph at bilbo.chm.uri.edu then you use the constant 7,1 × 10.
Write a chemical equation. The first, determine the ionic separation pattern of this compound when dissolved. Then write the equation with Ksp on one side and component ions on the other side.
- For example, a PbI molecule2 dissociate into ions Pb, I, and I. (You only need to know or check the charge of an ion, since all compounds are always electrically neutral).
- Write the equation 7,1 × 10 =
- This equation is the solubility constant, you can find out for 2 ions in the solubility chart. Since there are 2 l- ions, l- must be quadratic.
Transform equations to use variables. Rewrite the equation using normal algebraic methods, using the information you know about the number of molecules and ions. Set x equal to the mass of the compound to dissolve, and rewrite the equation where x represents the number of each ion.
- In this example, we need to rewrite the equation 7,1 × 10 =
- Since there is only one lead ion (Pb) in the compound, the number of molecules dissolved will be equal to the number of free lead ions. Hence we can set it to x.
- Since there are two iodine ions (I) for each lead ion, we set the number of iodine atoms equal to 2x.
- Now the equation becomes 7.1 × 10 = (x) (2x)
Take into account common ions, if any. Skip this step if you are dissolving the compound in distilled water. If a compound is dissolved in a solution that already has one or more component ions ("common ions"), the solubility of the compound will decrease significantly. The effect of the general ions will be most obvious on almost insoluble compounds, and in this case you can assume that most of the ions at equilibrium are those that were previously in solution. Rewrite the equation to calculate the molar concentration (mol per liter or M) of the ions already in the solution, replacing this value with the variable x you use for that ion.
- For example, if the lead iodide compound is dissolved in 0.2M lead chloride (PbCl) solution2), we will rewrite the equation as 7.1 × 10 = (0.2M + x) (2x). Since 0.2M is a higher concentration than x, we could rewrite it to 7.1 × 10 = (0.2M) (2x).
Solve the equation. Solve for x, and you will see the solubility of the compound. In the definition of the solubility constant, you must write your answer in terms of the number of moles of the compound dissolved per liter of water. You may have to use a computer to find the final answer.
- The following example is the solubility in distilled water without any common ions.
- 7.1 × 10 = (x) (2x)
- 7.1 × 10 = (x) (4x)
- 7.1 × 10 = 4x
- (7,1 × 10) ÷ 4 = x
- x = ∛ ((7,1 × 10) ÷ 4)
- x = 1,2 x 10 moles per liter will dissolve. This is a very small mass, so this compound is almost insoluble.
What you need
- Table of solubility product constants of the compound (Ksp)
Advice
- If you have experimental data on the amount of compounds dissolved, you can use the same equation to solve for the solubility constant K.sp.
Warning
- There is no consensus on the definitions of these terms, but chemists agree on the majority of the compounds. A number of special compounds in which both soluble and insoluble molecules make up significant constituents, each with a different description of these compounds.
- Some old textbooks see NH4OH is a soluble compound. This is not true; Small amounts of NH ions were detected4 and OH but these two ions cannot combine into compounds.