Author:
Lewis Jackson
Date Of Creation:
5 May 2021
Update Date:
1 July 2024
Content
Mol is the standard unit of measurement in chemistry, used to look at the different elements in a compound. Usually compound mass is calculated in grams (g) and needs to be converted to molar units. The transition is quite simple, however, there are still some important steps that we need to follow. Using the method below, you can convert grams to moles easily.
Steps
Part 1 of 2: Calculate molecular mass
Prepare the necessary supplies to solve the math problem. When you have all the tools available, it will be easier to solve the problem. What you need is:
- Pencil and paper. The math gets easier when you write everything down on paper. You need to present all the steps in order to reach the maximum score.
- Periodic table of chemical elements: used to determine the mass atoms of the elements.
- Pocket calculator: used to calculate complicated numbers.
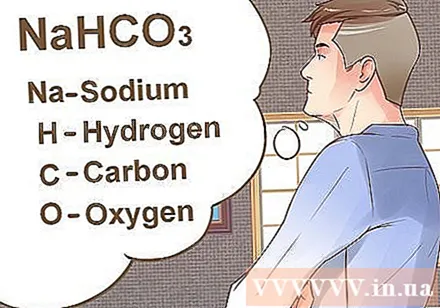
Determine which elements are in the compound you need to convert to molar units. The first step in calculating molecular mass is to determine the elements that make up the compound. This is easy because the abbreviation for elements is only one to two characters.- If a substance is abbreviated with two letters, the first letter will be capitalized and the second letter is lowercase. Example: Mg is the abbreviation for the element magnesium.
- NaHCO compounds3 consists of four elements: sodium (Na), hydrogen (H), carbon (C) and oxygen (O).
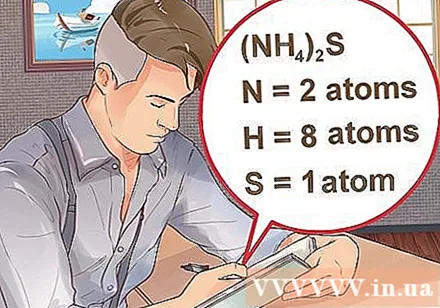
Determine the number of atoms for each element in the compound. You need to know the atomic number of each substance in a compound to calculate the mass molecule of that compound. The small number next to the element's initials represents the element's atomic number.- Example: compound H2O has two hydrogen atoms and one oxygen atom.
- If a compound is written in parentheses, followed by a small index, each ingredient in parentheses multiplies the index. Example: compound (NH4)2S consists of two N atoms, eight H atoms and one S atom.
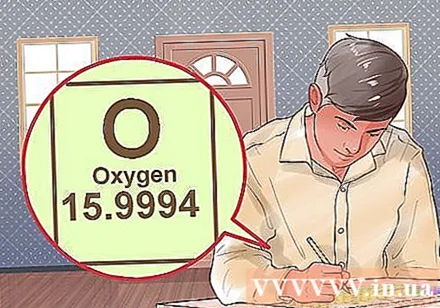
Write down the cubic atomic paper for each element. Using the periodic table is the easiest way to find the cubic atom of an element. After you locate the element on the periodic table, you will see the atomic mass just below the element's icon.- For example, the cubic atom of oxygen is 15.99.
Calculate the molecular mass. The mass molecule of a substance is equal to the number of atoms of each element multiplied by the mass atom of that element. This quantity is essential in gram to molar conversion.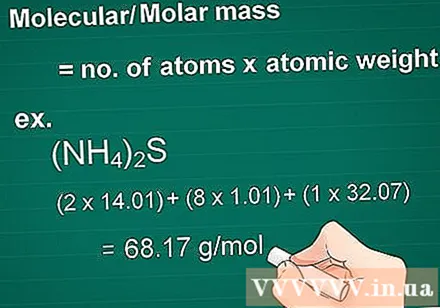
- First, multiply the atomic number of each element in the compound by its mass atom.
- Then, add the masses of the elements in the compound together.
- Example: Molecular mass of a compound (NH4)2S = (2 x 14.01) + (8 x 1.01) + (1 x 32.07) = 68.17 g / mol.
- Molecular mass is also known as molar mass.
Part 2 of 2: Convert grams to mol
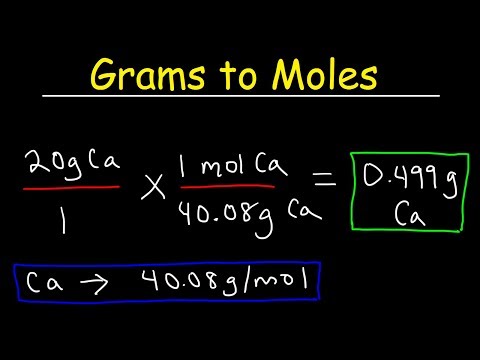
Set up the conversion formula. To find the number of moles of the compound, divide the number of grams of the compound by the molar mass of that compound.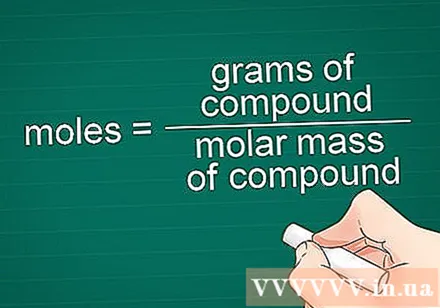
- Formula: number of moles = mass (grams) / molar mass of compound (gram / mol)
Substitute the numbers into the formula. After setting up the right formula, the next step is to replace the numbers you calculated with your formula. If you want to make sure that the data is in the correct position, you can check it by suppressing the unit. If after simplicity the remaining unit is moles then you are set correctly.
Solve the equation. Using a calculator, divide the mass by the cubic molecule of the substance or compound. The quotient will be the number of moles of the substance or compound you are looking for.
- For example, the problem is for 2 g of water (H2O) and ask you to convert it to molar units. We have the molar mass of H2O is 18g / mol. Divide 2 by 18, so you have 0.1111 mol H2O.
Advice
- Don't forget to include the element or compound name with the answers.
- If you are asked to present an exercise or quiz, make sure you show your answers clearly by circling or drawing a box around the answers.
What you need
- Chemical periodic table
- Pencil
- Paper
- Computer
- Chemistry problems